Denaturation
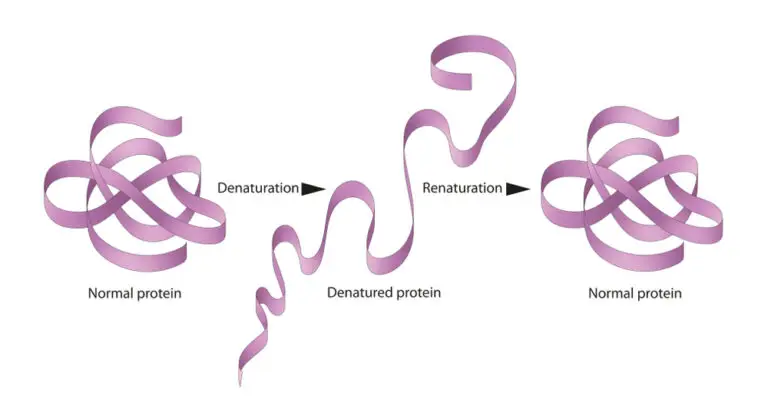
Table of Contents
What is Denaturation?
Denaturation refers to the alteration or disruption of the structure of a biological molecule, such as proteins or nucleic acids, without breaking their covalent bonds. This process often results in losing the molecule’s original structure and, consequently, its biological activity.
Various factors, including heat, can induce denaturation, changes in pH, or exposure to certain chemicals.
Denaturation Overview
Protein Denaturation
- Temperature: Elevated temperatures can lead to protein denaturation. Heating a protein disrupts the weak bonds (hydrogen bonds, van der Waals forces) and other non-covalent interactions that maintain the protein’s three-dimensional structure.
- pH: Extreme changes in pH can alter the charge distribution in a protein, affecting its structure and function.
- Chemicals: Certain chemicals, such as urea and guanidine hydrochloride, can denature proteins by disrupting their native structure.
Nucleic Acid Denaturation
- Temperature: Nucleic acids, such as DNA and RNA, can undergo denaturation through heating. This process is often reversible and is the basis for techniques like polymerase chain reaction (PCR), where DNA strands are temporarily separated to facilitate replication.
- Chemicals: Denaturing agents like formamide or certain detergents can also induce nucleic acid denaturation.
Effects on Biological Activity
Denaturation typically results in the loss of the original biological activity of the molecule.
For proteins, denaturation may lead to the loss of enzymatic activity or the inability to bind to specific substrates. In nucleic acids, denaturation may disrupt the base pairing essential for processes like DNA replication and transcription.
Reversibility
The reversibility of denaturation depends on the extent and nature of the denaturing conditions. Some proteins and nucleic acids can refold and regain their native structure if the denaturing conditions are mild and reversible.
Applications
Denaturation and renaturation processes are utilized in various laboratory techniques. For example, DNA denaturation is a key step in Southern blotting and hybridization experiments. In cooking, denaturation contributes to the changes in the texture and structure of food proteins.
Biological Significance
- In living organisms, maintaining the proper structure of proteins and nucleic acids is crucial for their normal function.
- Denaturation can be a protective mechanism under stress conditions, preventing irreversible damage to biomolecules.
Related Links
Protein
Urea
Nucleotide
Deoxyribose