Proton (Biology)
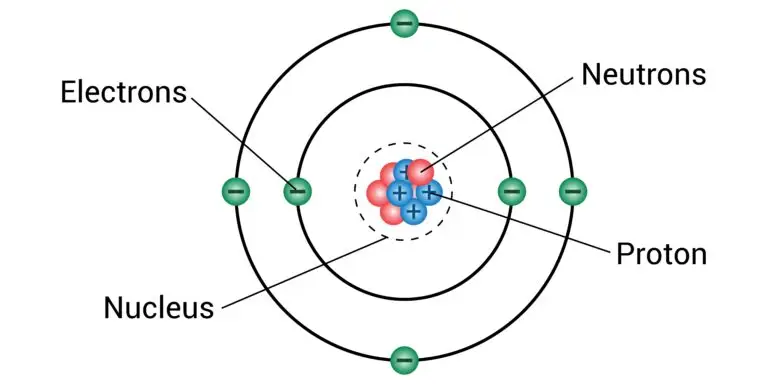
Table of Contents
What is a Proton?
A proton is a subatomic particle found in an atom’s nucleus. It carries a positive electric charge and is one of the building blocks of matter. Protons, along with neutrons, make up the nucleus of an atom, which is the central core where most of an atom’s mass is concentrated.
Characteristics of Protons
Charge
Protons carry a positive electric charge. The elementary charge of a proton is approximately +1+1.
Mass
The mass of a proton is approximately 1.6726219×10−271.6726219×10−27 kilograms. This mass is roughly 1,836 times the mass of an electron.
Location
Protons are located in the nucleus of an atom. The nucleus is the central region of the atom, composed of protons and neutrons.
Stability
Protons are relatively stable particles. They are not subject to radioactive decay within the nucleus.
Role in Atomic Structure
Along with neutrons, protons contribute to the mass of an atom. The number of protons in an atom determines its atomic number and, consequently, its identity as a specific element.
Atomic Number
The atomic number of an element is equal to the number of protons in the nucleus of an atom of that element. It is a unique identifier for each element on the periodic table.
Quarks
Protons are composed of elementary particles called quarks. Specifically, protons consist of three quarks: two “up” quarks and one “down” quark.
Related Links
Atom
Mass Number
Neutrons
Replication