Base (Chemistry)
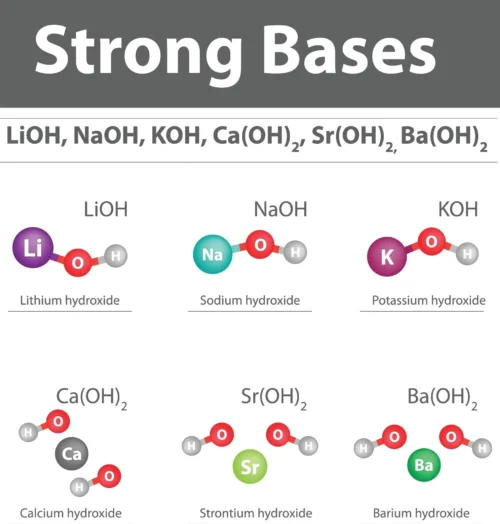
Table of Contents
What is a Base in Chemistry?
A base is like a “chemical helper” that plays an important role in reactions. Bases have a specific ability: they can either accept protons (H+) or donate electrons during a chemical reaction. This ability makes them essential players in the world of chemistry.
One of the key characteristics of bases is their ability to neutralize acids. When a base reacts with an acid, it undergoes a chemical reaction that forms water (H2O) and a salt. This process is known as neutralization, where the acidic and basic properties cancel each other out, leaving behind a neutral solution.
For example, if you mix hydrochloric acid (HCl) with a base like sodium hydroxide (NaOH), it will react to form water (H2O) and a salt called sodium chloride (NaCl). The sodium hydroxide (base) accepts the proton from hydrochloric acid (acid), and in the process, water and sodium chloride are produced.
All About The Base
Proton Acceptance
Bases play a critical role as proton acceptors in chemistry. When they encounter acids, bases accept protons (H+) from the acid molecules. This interaction leads to a chemical reaction known as neutralization, where the base and acid components balance each other’s properties.
During neutralization, the proton transfer between the base and acid forms water (H_2O) and salt. The water molecules are produced by combining hydrogen ions (H+) from the acid and hydroxide ions (OH-) from the base. Meanwhile, the remaining components from the acid and base combine to form a salt.
For example, if you mix hydrochloric acid (HCl) with a base like sodium hydroxide (NaOH), the base (NaOH) will accept the proton (H+) from the acid (HCl). This proton transfer leads to water (HO) and salt sodium chloride (NaCl) formation.
HCl (acid)+NaOH (base)→H2O (water)+NaCl (salt)HCl (acid)+NaOH (base)→H_2O (water)+NaCl (salt)
This neutralization process results in a solution with a pH closer to neutral (pH 7). In essence, bases are crucial in neutralizing acidic solutions, forming less acidic or neutral solutions that are often less reactive and more suitable for various applications.
Arrhenius Definition
According to the Arrhenius definition, bases are substances that, when dissolved in water, increase the concentration of hydroxide ions (OH-) in the solution. This definition helps us understand the behavior of bases in aqueous environments.
When a base, such as sodium hydroxide (NaOH) or calcium hydroxide (Ca(OH)_2), is added to water, it dissociates into ions. For example, sodium hydroxide breaks down into sodium ions (Na+) and hydroxide ions (OH-) in water:
NaOH (sodium hydroxide)→Na+ (sodium ion)+OH- (hydroxide ion)NaOH (sodium hydroxide)→Na+ (sodium ion)+OH- (hydroxide ion)
The hydroxide ions (OH-) released by the base increase the concentration of these ions in the solution. This increase in hydroxide ions contributes to the alkalinity of the solution, making it basic.
Common examples of Arrhenius bases include metal hydroxides like sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)_2). These substances are widely used in various applications, such as household cleaning products, manufacturing processes, and water treatment, due to their ability to increase hydroxide ion concentration and neutralize acids.
Bronsted-Lowry Definition
In the Bronsted-Lowry theory, bases are substances capable of accepting a proton (H+) from another substance. This definition is broader than the Arrhenius definition because it includes hydroxide ions and other species that can accept protons.
When a base accepts a proton, it forms a new substance called the conjugate acid. This proton transfer process is at the heart of the Bronsted-Lowry theory of acids and bases.
For example, consider ammonia (NH_3). Ammonia acts as a base because it can accept a proton (H+) to form its conjugate acid, ammonium ion (NH_4+):
NH_3 (Ammonia) + H + \to NH_4 (ammonium ion)
Similarly, the bicarbonate ion (HCO_3-) can also act as a base by accepting a proton to form its conjugate acid, carbonic acid (H_2CO_3):
HCO[latex_3][/latex]– (bicarbonate ion) + H + \to H_2CO_3
Lewis Theory
In Lewis’s theory, bases are substances that can donate a pair of electrons to form a covalent bond with another species, often an acid. This definition expands the concept of bases beyond proton acceptors and includes species that can donate electron pairs.
A classic example of a Lewis base is ammonia (NH_3). Ammonia has a lone pair of electrons on its nitrogen atom, which it can donate to form a covalent bond with another species that can accept these electrons, such as a Lewis acid. The donation of the electron pair from ammonia to the Lewis acid forms a new molecule or complex.
Similarly, water (H2O) is also a Lewis base because it has two lone pairs of electrons on its oxygen atom. Water can donate one of these lone pairs to form a covalent bond with a species that can accept the electrons, acting as a Lewis acid.
H2O (water)+Lewis Acid→New Molecule or Complex
Properties of Bases
Bases exhibit several characteristic properties that help identify them in chemical reactions and everyday situations. These properties include a bitter taste, a slippery or soapy feel, and the ability to turn red litmus paper blue.
One of the distinctive features of bases is their bitter taste, although this characteristic is not often tested due to safety concerns. Bases also feel slippery or soapy when touched, resulting from their reaction with oils and fats on the skin to form soap-like compounds.
Another important property of bases is their ability to change the color of certain indicators. For example, bases turn red litmus paper blue. Litmus paper is a common pH indicator that changes color depending on whether a solution is acidic (red) or basic (blue). When a base is added to red litmus paper, it causes the paper to turn blue, indicating the presence of a base.
Application of Bases
One prominent base, sodium hydroxide (NaOH), is found to be extensively used in soap and detergent production. Its alkaline properties help in saponification, converting fats and oils into soap. Sodium hydroxide is also vital in water treatment processes, where it neutralizes acidic impurities to purify water for consumption and industrial use. Additionally, sodium hydroxide is employed in food processing, especially for pH adjustment and as a leavening agent in baked goods.
Another essential base is calcium hydroxide (Ca(OH)2), slaked or hydrated lime. In agriculture, calcium hydroxide is a soil amendment to adjust soil pH and improve plant nutrient availability. It also produces lime mortar and plaster, contributing to the construction industry by providing durable and weather-resistant building materials.
Ammonia (NH3) is a versatile base with widespread applications. In agriculture, it is a key component of fertilizers, providing essential nitrogen nutrients for plant growth. Ammonia is also used in cleaning products for its alkaline properties, effectively removing stains and grease. Furthermore, ammonia is a refrigerant in industrial and commercial refrigeration systems, contributing to efficient cooling processes.
pH Scale
Bases are substances that typically have pH values greater than 7 on the pH scale, indicating their alkalinity. The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution based on the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in the solution.
On the pH scale, values below 7 indicate acidity, with lower numbers representing stronger acids. Values above 7 indicate alkalinity, with higher numbers representing stronger bases. For example, a pH of 8 is slightly basic, while a pH of 11 is moderately basic, and a pH of 14 is strongly basic.
The pH scale is logarithmic, meaning each whole number change on the scale represents a tenfold difference in the concentration of hydrogen ions. For instance, a solution with a pH of 8 has 10 times fewer hydrogen ions than a solution with a pH of 7, while a solution with a pH of 11 has 100 times fewer hydrogen ions than a solution with a pH of 9.
Related Links
Acid
Chemical Bond
Chemical Compound
Element Groups