Electrons
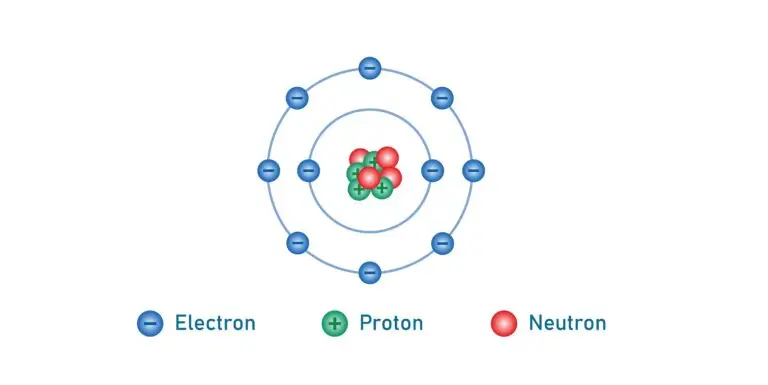
Table of Contents
What are Electrons?
Electrons are tiny, negatively charged subatomic particles that are essential components of atoms. These negatively charged particles are crucial for the stability and behavior of atoms.
Electron Properties
Location and Orbitals
Electrons are positioned outside the central nucleus of an atom, and they inhabit specific regions known as orbitals or electron shells. These shells are arranged in energy levels, with the innermost shell closest to the nucleus having the lowest energy level.
Each successive shell has a higher energy level as you move outward. This organization of electron shells determines the atom’s stability and how it interacts with other atoms to form molecules.
Charge and Mass
Electrons possess a negative electrical charge, represented as -1, which balances an atom’s positive charge of protons (+1). This negative charge is equal in magnitude to the positive charge of protons but opposite in polarity.
Additionally, electrons have a significantly smaller mass than protons and neutrons, with an approximate mass of 9.109 x 10^-31 kilograms. This lightweight nature of electrons is a fundamental aspect of atomic structure and plays a crucial role in determining the properties and behavior of atoms.
Quantum Numbers
Electrons are described by quantum numbers that specify their energy, orbital shape, orientation, and spin. The principal quantum number (n) indicates the energy level of the electron, while the azimuthal quantum number (l) determines the shape of the orbital (s, p, d, f).
The magnetic quantum number (m_l) specifies the orbital’s orientation in space, and the spin quantum number (m_s) indicates the electron’s spin direction (+1/2 or -1/2).
Electron Configuration
The organization of electrons within an atom’s electron shells is known as its electron configuration. This configuration follows particular rules, including:
Aufbau Principle: Electrons fill orbitals and energy levels, starting from the lowest and moving to higher energy levels sequentially.
Pauli Exclusion Principle: No two electrons within an atom can have the same set of quantum numbers, which means they must have opposite spins.
Hund’s Rule: When multiple orbitals of the same energy level are available, electrons occupy them singly before pairing up. This rule ensures maximum electron spread and minimizes electron-electron repulsion.
These rules guide how electrons are distributed among the various orbitals and energy levels in an atom, ultimately determining the atom’s stability, reactivity, and chemical properties.
Electrons Role in Chemistry
Electrons are incredibly important in the realm of chemistry, particularly in the formation of chemical bonds and reactions. They engage in various bonding processes:
Covalent Bonds: Electrons are shared between atoms to create covalent bonds. This sharing of electrons leads to stable molecules and compounds, such as water (H2O) and methane (CH4).
Ionic Bonds: In ionic bonding, electrons are transferred from one atom to another, forming positive and negative ions that attract each other. This leads to the creation of ionic compounds like sodium chloride (NaCl) and calcium carbonate (CaCO3).
Metallic Bonds: In metallic bonding, electrons are delocalized and free to move throughout a metal lattice. This results in metals having properties like electrical conductivity and malleability.
The arrangement of electrons in an atom’s electron shells is crucial because it determines the atom’s chemical properties and reactivity. For instance, elements with incomplete electron shells are often highly reactive, seeking to gain or lose electrons to achieve a stable electron configuration.
Electron Cloud Model
The electron cloud model is a conceptual framework in quantum mechanics that describes electrons as existing in a space around the nucleus called an electron cloud.
This model acknowledges the uncertainty principle, a fundamental concept in quantum physics. According to the uncertainty principle, it’s impossible to precisely determine both the position and momentum of a particle simultaneously.
Therefore, instead of having fixed orbits like planets around the sun, electrons in atoms are represented as being distributed within a three-dimensional space, forming a cloud-like structure where the probability of finding an electron is highest. This probabilistic nature of electron distribution is a key aspect of understanding atomic structure and behavior at the quantum level.
Energy Levels and Spectroscopy
Electrons can absorb or release energy in specific quantities, known as quanta when they move between different energy levels or orbitals within an atom. This phenomenon forms the foundation of spectroscopy techniques essential for studying the energy states, electronic structure, and characteristics of atoms and molecules.
In spectroscopy, scientists analyze the interactions between matter and electromagnetic radiation, such as light. Electromagnetic radiation interacting with atoms or molecules can cause electrons to transition between energy levels. These transitions result in the absorption or emission of light energy at specific wavelengths corresponding to the energy difference between the electrons’ initial and final energy levels.
By measuring the absorbed or emitted light at different wavelengths, spectroscopy provides valuable information about the energy levels, electronic configurations, and properties of atoms and molecules.
Related Links
Atomic Number
Chemical Bond
Mass Number
Protons (Chemistry)