Element Groups
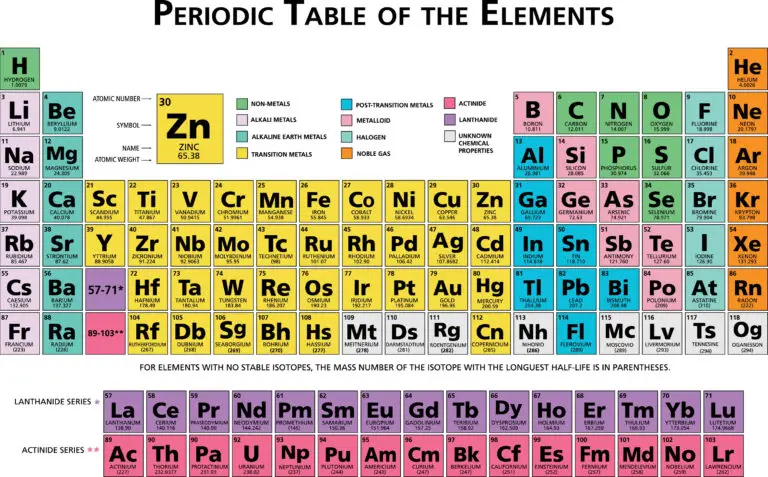
Table of Contents
Element Group Overview
Element groups are arranged on the periodic table based on similarities in their chemical properties, electron configurations, and the number of valence electrons they have.
Each group comprises elements that exhibit comparable reactivity and bonding behaviors. This organization allows scientists to predict how elements will interact with one another and form compounds based on their placement in the periodic table.
Elements in the same group often share similar properties due to their analogous electron configurations and valence electron numbers, making studying and understanding their chemical behavior easier.
The 18 Element Groups
Alkali Metals (Group 1)
The group known as alkali metals encompasses elements such as lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These metals are characterized by their high reactivity and tendency to readily give up their one valence electron, forming positively charged ions with a +1 charge, known as cations.
Alkali metals exhibit softness, low melting points, and low densities compared to many other metals. When they come into contact with water, alkali metals react vigorously, producing alkaline solutions and releasing hydrogen gas.
Alkaline Earth Metals (Group 2)
The alkaline earth metals include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
These metals have two valence electrons and commonly form +2 ions (cations) in chemical reactions. They are less reactive than alkali metals but exhibit reactivity, especially with water and acids.
Transition Metals (Groups 3-12)
Transition metals are characterized by their variable oxidation states and the formation of colorful compounds. This group includes metals like iron (Fe), copper (Cu), zinc (Zn), silver (Ag), and gold (Au).
Transition metals often exhibit high melting points, conductivity, and malleability. They are used extensively in industry, technology, and manufacturing.
Halogens (Group 17)
The halogens group includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements are characterized by having seven valence electrons, making them highly reactive nonmetals.
In chemical reactions, halogens readily gain one electron to form negatively charged ions with a -1 charge, known as anions. They often react with metals to create salts and are commonly used in disinfectants, bleaches, and pharmaceuticals due to their reactivity and ability to form compounds with various elements.
Noble Gases (Group 18)
The noble gases group comprises helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These gases are called “noble” because they have full outer electron shells, making them highly stable and unreactive under typical conditions. They are colorless and odorless and are often used in specialized applications.
For instance, neon is used in neon lights for its bright color, while argon and helium are used in inert gas atmospheres and cryogenics due to their stability and low reactivity.
Metalloids (Semimetals)
Metalloids are a group of elements that display properties between metals and nonmetals. They are positioned along the zigzag line on the periodic table and include elements such as silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te).
Metalloids possess characteristics of both metals and nonmetals, such as being able to conduct electricity to some degree. This property makes them crucial in semiconductor technology, where they are utilized in producing electronic devices like computer chips and solar cells.
Rare Earth Elements
Rare earth elements are a special group encompassing scandium (Sc), yttrium (Y), and the lanthanides and actinides series. These elements are often categorized separately because of their distinct electronic configurations and properties.
Rare earth elements find wide-ranging applications in various fields, such as technology, electronics, magnets, and catalysis. Their unique characteristics make them indispensable in the development of advanced materials, devices, and processes across different industries.
Related Links
Alkali Metals
Alkaline Earth Metals
Halogens
Period (Periodic Table)