Molecule
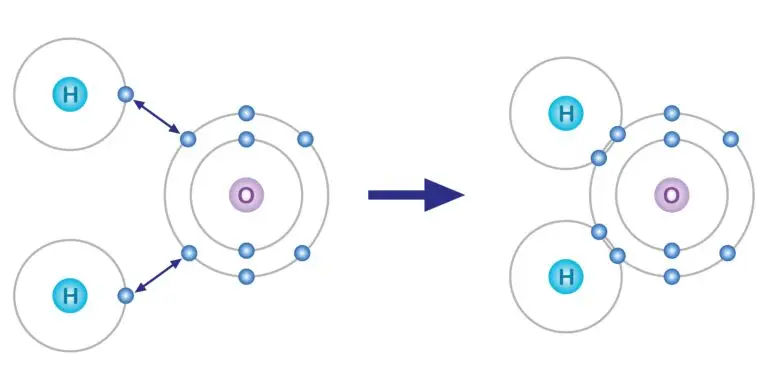
Table of Contents
What is a Molecule
A molecule is formed when two or more atoms bond chemically, creating a stable and electrically neutral entity. These atoms can be from the same element, leading to molecules like O₂ (oxygen gas), where both atoms are oxygen.
They can also be from different elements, resulting in compounds like H₂O (water), which contains hydrogen and oxygen. The chemical bond that holds the atoms together in a molecule is the force that maintains the molecule’s stability and neutrality.
The Role of Molecules
Composition
Molecules consist of atoms bonded together through chemical forces. These atoms can either belong to the same element or different elements. For example, diatomic molecules such as O₂ (oxygen), N₂ (nitrogen), and H₂ (hydrogen) are made up of two atoms of the same element, forming some of the basic building blocks of the Earth’s atmosphere and biological processes.
On the other hand, molecules like water (H₂O) are composed of atoms from different elements, in this case, two hydrogen atoms and one oxygen atom, resulting in a compound with properties distinct from those of its constituent elements. This variety in molecular composition underpins the diversity of chemical substances and materials in the natural and synthetic worlds.
Chemical Bonds
Atoms in a molecule are held together by chemical bonds, which can be covalent, ionic, or metallic, each type having its mechanism for forming stable structures. Covalent bonds occur when atoms share electrons, allowing them to achieve a stable electron configuration. This type of bonding is common in organic compounds, such as water (H₂O) and carbon dioxide (CO₂), where the shared electrons create a strong bond between the atoms.
On the other hand, Ionic bonds form through the transfer of electrons from one atom to another, creating positively and negatively charged ions. This electrostatic attraction between oppositely charged ions holds them together, as seen in table salt (NaCl), where sodium (Na) and chlorine (Cl) form a bond.
Metallic bonds are characterized by delocalized electrons flowing around a lattice of metal ions. These electrons are not associated with any specific atom and can move freely, which allows metals to conduct electricity and heat efficiently. This bonding gives metals their distinctive properties, such as malleability and ductility.
Molecule Properties
Molecules exhibit properties that are distinct from those of their constituent atoms, due to the specific ways in which atoms are arranged and interact within the molecule. Physical properties like melting point, boiling point, and density arise from the molecular structure and the strength of the bonds holding the atoms together. For example, water has a high boiling point relative to its molecular weight due to the strong hydrogen bonds between water molecules.
Chemical properties, such as reactivity, acidity/basicity, and solubility, are also determined by the molecular structure and the type of chemical bonds within the molecule. The atoms’ arrangement and the chemical bonds’ nature influence how a molecule interacts with other substances.
For instance, the acidity of a molecule like hydrochloric acid (HCl) is due to a hydrogen ion (H⁺) that can be easily donated in a chemical reaction. Similarly, the solubility of a compound in water or other solvents is influenced by the molecule’s ability to form favorable interactions, like hydrogen bonds or ionic interactions, with the solvent.
Structural Formulas
Molecules are depicted through structural formulas that illustrate the arrangement of atoms and the bonds connecting them within the molecule. These representations provide a visual insight into the molecule’s structure, showing how the atoms are linked and the type of bonds formed between them. In the case of water (H₂O), its structural formula represents one oxygen atom bonded to two hydrogen atoms. Each hydrogen atom is connected to the oxygen atom via a single covalent bond, indicating the sharing of electrons between the oxygen and hydrogen atoms.
This depiction helps in understanding the molecular geometry and the spatial arrangement of atoms, which are crucial for predicting the physical and chemical properties of the molecule. The structural formula for water, for instance, highlights the bent shape of the molecule, which is key to its unique properties, such as its high boiling point and ability to dissolve various substances.
Functional Groups
In organic chemistry, functional groups are crucial because they define the chemical behavior of molecules. These groups are specific clusters of atoms within a molecule that confer distinct chemical properties and reactivity patterns. For instance, the hydroxyl group (-OH) is a defining feature of alcohols, giving them their characteristic properties, such as solubility in water and reactivity with acids to form esters.
The carboxyl group (-COOH) is another important functional group, found in carboxylic acids. It consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group. This arrangement makes carboxylic acids typically acidic and reactive in condensation reactions. Similarly, the amino group (-NH2) is a fundamental component of amines, affecting their basic nature and making them reactive towards acids to form amides.
Role in Chemistry
Molecules are the cornerstone of chemistry and are pivotal in understanding the intricacies of chemical reactions, bonding, and the structure-function relationships within compounds.
They are the smallest units of a compound that retain chemical properties and are key to determining how substances interact, react, and change under different conditions. In chemical reactions, molecules interact with each other, forming new compounds through the breaking and forming of chemical bonds, which are the basis for changes in matter and energy.
Types of Molecules
Simple Molecules
Simple molecules consist of just one type of atom bonded together, forming diatomic or monoatomic structures. Oxygen gas (O₂), nitrogen gas (N₂), and hydrogen gas (H₂) are classic examples of simple molecules where two atoms of the same element are bonded to each other.
These molecules are fundamental to various biological and chemical processes. For instance, O₂ is essential for respiration in most living organisms, N₂ makes up a significant part of Earth’s atmosphere and plays a key role in the nitrogen cycle, and H₂ is involved in many industrial processes, including the production of ammonia for fertilizers and as a potential clean fuel source.
Compound Molecules
Molecules made up of atoms from different elements bonded together are known as compound molecules. Water (H₂O), carbon dioxide (CO₂), and methane (CH₄) are prime examples of this category. In these molecules, the atoms are joined through chemical bonds, forming substances with properties distinct from those of their constituent elements.
For instance, water, a compound of hydrogen and oxygen, is vital for all known forms of life and has unique properties like high heat capacity and solvent capabilities. Carbon dioxide, composed of carbon and oxygen, plays a key role in Earth’s carbon cycle and is a significant greenhouse gas affecting global climate. Methane, a compound of carbon and hydrogen, is both a major energy source as natural gas and a potent greenhouse gas.
Polyatomic Molecules
Polyatomic molecules are composed of more than two atoms, often consisting of multiple elements bonded together, forming complex structures. Sulfuric acid (H₂SO₄), ammonia (NH₃), and glucose (C₆H₁₂O₆) are examples of such molecules.
Sulfuric acid, with two hydrogen atoms, one sulfur atom, and four oxygen atoms, is a highly corrosive substance and a key industrial chemical used in the production of fertilizers, explosives, and in chemical manufacturing processes. Ammonia, made up of one nitrogen atom and three hydrogen atoms, is crucial in the synthesis of various organic compounds and is a fundamental building block for the production of fertilizers.
Glucose, a sugar molecule with six carbon atoms, twelve hydrogen atoms, and six oxygen atoms, is essential for life, serving as a primary energy source for cells.
Related Links
Atom
Base Pair
Chemical Compound
Electrons