Neutrons
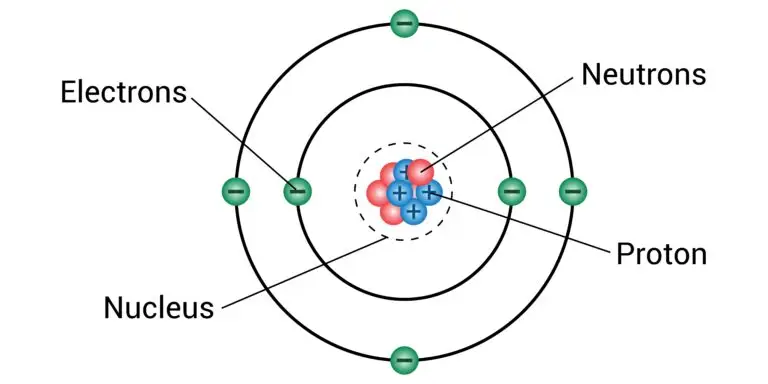
Table of Contents
What is a Neutron
A neutron is a subatomic particle located in the nucleus of an atom alongside protons. Neutrons are electrically neutral, which means they carry no net charge.
Unlike protons, which have a positive charge (+1), and electrons, which have a negative charge (-1), neutrons do not affect an atom’s electrical charge. This neutrality is crucial for the nucleus’s stability, as neutrons help to balance the repulsive forces between positively charged protons, preventing the nucleus from breaking apart.
Attributes of Neutrons
Composition
Neutrons, together with protons, form the core of an atomic nucleus. Protons carry a positive electrical charge, whereas neutrons are electrically neutral, having no charge.
This neutrality of neutrons is vital for the stability of the nucleus, as it helps to offset the repulsive forces between the positively charged protons. In terms of mass, neutrons are slightly heavier than protons, which contributes to the overall mass of an atom.
This slight difference in mass between neutrons and protons is important in nuclear physics, affecting the behavior and stability of atoms. The balance of neutrons and protons determines the nucleus’s stability and the atom’s isotopic identity.
Symbolic Representation
Neutrons are represented symbolically by “n” or “N” and can be denoted as “1n” or simply “n” to indicate their presence in atomic notation. For example, the most common isotope of hydrogen, known as hydrogen-1 (^1H), has one proton and no neutrons in its nucleus. In contrast, deuterium, an isotope of hydrogen, is represented as \Large ^\frac{2}{1}H or \Large^\frac{2}{1}D, indicating it has one proton and one neutron, giving it a mass number of 2. This notation helps to clearly distinguish between different isotopes of an element, showing the number of protons and neutrons in the nucleus.
Role in Atomic Structure
Neutrons are vital for the stability of the atomic nucleus because they mitigate the electrostatic repulsion between the positively charged protons. This repulsion occurs because, like charges, they repel each other, and without neutrons, the protons would push each other apart, leading to an unstable nucleus. Neutrons contribute an attractive force that acts over short distances, known as the strong nuclear force, one of nature’s four fundamental forces.
The strong nuclear force is much more powerful than the electrostatic repulsive force at the distances within the nucleus, enabling it to hold the protons and neutrons together tightly. This force ensures that the nucleus remains stable and intact, preventing it from disintegrating due to the repulsive forces among the protons.
Isotopes
Neutrons significantly impact the mass number of an atom, which is calculated by adding the number of protons and neutrons in the nucleus. Since isotopes of an element have identical numbers of protons (and thus the same atomic number), they differ in their neutron count, resulting in different atomic masses.
For instance, carbon-12 (^{12}C) contains 6 protons and 6 neutrons, totaling a mass number of 12. Carbon-13 (^{13}C) has the same 6 protons but with 7 neutrons, leading to a mass number of 13. Similarly, carbon-14 (^{14}) also has 6 protons but with 8 neutrons, resulting in a mass number of 14.
These variations in the number of neutrons among the isotopes of carbon cause differences in their atomic masses, highlighting the role of neutrons in determining the mass and form of different isotopes.
Nuclear Reactions
Neutrons play a key role in nuclear reactions, including nuclear fission and fusion, which release a significant amount of energy. In nuclear fission, which occurs in nuclear reactors, neutrons initiate the reaction by bombarding fissile materials such as uranium-235 or plutonium-239.
When a neutron collides with the nucleus of a fissile atom, it can cause the nucleus to become unstable and split into two smaller nuclei, releasing more neutrons and a large amount of energy in the process. This chain reaction is the basis of nuclear power generation.
In nuclear fusion, the process that powers stars, including the sun, neutrons are produced as a byproduct. Fusion involves the combination of light atomic nuclei, such as hydrogen isotopes, to form heavier nuclei. This process occurs under extremely high temperatures and pressure, releasing tremendous energy, part of which is in the form of neutrons.
For instance, in the fusion of deuterium and tritium (isotopes of hydrogen), the reaction yields a helium nucleus and a neutron, releasing significant energy. This energy release in fusion is much greater per unit of mass than in fission, making fusion a potent source of energy. However, it is currently more challenging to control and utilize for practical purposes.
Neutron Decay
Neutrons are stable within the environment of an atomic nucleus, where they are bound together with protons by the strong nuclear force. However, once outside the nucleus, free neutrons are unstable and undergo a process known as beta decay. In beta decay, a neutron transforms into a proton, an electron (often referred to as a beta particle), and an antineutrino. This transformation increases the atomic number of an element by one, changing the element itself in the process.
The beta decay of neutrons is mediated by the weak nuclear force, one of the four fundamental forces in physics, which is responsible for radioactive decay processes. During this decay, a down quark in the neutron converts into an up quark, resulting in the change from a neutron to a proton. The emission of the electron and antineutrino in this process helps to conserve charge, lepton number, and energy.
Related Links
Atom
Fusion
Isotopes
Radiation