Period (Periodic Table)
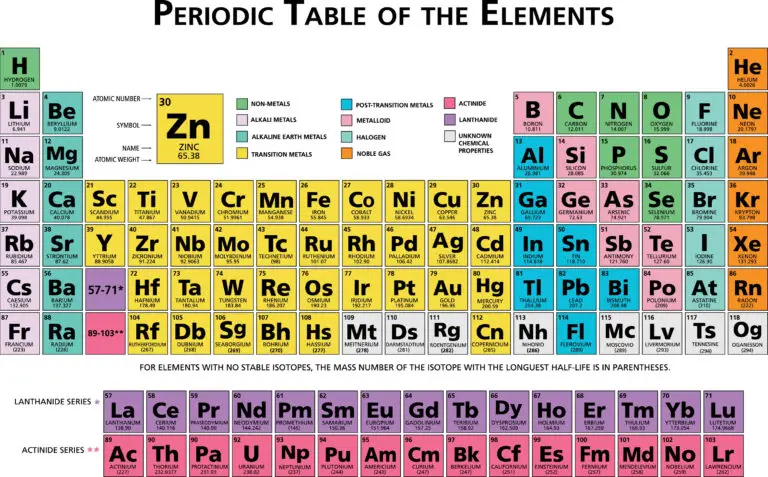
Table of Contents
Periodic Table Periods
A period is a horizontal row in the periodic table that represents a sequence of elements with the same number of electron shells, or energy levels, in their atoms. As you move from left to right across a period, each element has one more proton and electron than the element before it, leading to a gradual change in chemical and physical properties. These changes are systematic and predictable, reflecting the filling of electron shells and subshells according to the principles of quantum mechanics.
The length of each period varies because it corresponds to the filling of different electron shells, which can accommodate varying numbers of electrons. For instance, the first period contains only two elements, hydrogen, and helium, because the first shell can hold up to two electrons. Subsequent periods have more elements as the electron capacity of the shells increases. This arrangement in the periodic table organizes elements in a logical sequence and helps us understand the underlying principles of atomic structure and the behavior of elements.
Structure of Periods
Electron Shells
The organization of elements into periods on the periodic table is directly related to their atomic structure, particularly the number of electron shells or energy levels in their atoms. Elements within the same period share the same number of electron shells, but the number of electrons in these shells increases as you move from left to right across the period. This increase corresponds to the elements’ growing atomic number, which is the number of protons in their nuclei.
As we proceed from one period to the next in the periodic table, the number of electron shells increases. This means that elements in higher periods have more electron shells compared to those in lower periods. For example, all elements in the first period have one electron shell, while elements in the second period have two electron shells, and so on. This systematic increase in the number of electron shells as you move down the table helps to determine the chemical and physical properties of the elements, influencing their behavior in different environments and reactions.
Periodic Table Layout
The periodic table is structured into rows called periods and columns, known as groups or families. It comprises seven periods, numbered from 1 to 7, each representing a series of elements with increasing atomic numbers. Elements within the same period have the same number of electron shells, but their electron configurations vary progressively, leading to changes in their chemical and physical properties.
As you move across a period from left to right, the number of electrons in the outer shell of the elements increases, modifying their atomic properties. This arrangement reflects the periodic nature of the elements, where patterns in properties recur at regular intervals. Groups or families, on the other hand, consist of elements that have similar outer electron configurations and, therefore, exhibit similar chemical behaviors. For example, the elements in Group 1, also known as the alkali metals, all have a single electron in their outermost shell, making them highly reactive.
Transition Between Periods
The transition from one period to the next in the periodic table happens when electrons start to fill a new electron shell. This progression is evident in the gradual increase in atomic size across a period. As we move from left to right within a period, electrons are added to the same shell, slightly increasing the size of the atom initially due to increased electron-electron repulsion. However, the size then decreases towards the end of the period because the nucleus’s increasing positive charge pulls the electrons closer, outweighing the electron-electron repulsion.
When a new period begins, an electron is added to a new, outer shell, which increases the overall size of the atom. This addition of a new shell means that the electrons are further from the nucleus, leading to an increase in atomic size. Therefore, as you move down the periodic table from one period to the next, the atoms generally become larger because they have more electron shells, contributing to the larger radius of each successive element. This pattern repeats with each new period, reflecting the structured nature of atomic properties in the periodic table.
Representative Elements and Transition Metals
In the periodic table, the first two periods consist mainly of representative elements, also known as main group elements, found in groups 1, 2, and 13 to 18. These elements include alkali metals, alkaline earth metals, p-block elements, and noble gases, which are characterized by their varied and predictable chemical behaviors based on their valence electron configurations.
Starting from period 3, the table also includes transition metals, which occupy the central block of the table from groups 3 to 12 and span periods 4 to 7. Transition metals are distinguished by their ability to form various oxidation states and to form colored compounds, due to the d-subshell electrons that are involved in bonding and chemical reactions.
The presence of representative and transition metals in periods 3 to 7 reflects the complexity and diversity of chemical elements as the atomic number increases, showcasing a wide range of physical and chemical properties.
Periodic Table Blocks
The periodic table is divided into blocks that reflect the electron configurations of the elements, specifically which orbital types are being filled with electrons. These blocks are the s-block, p-block, d-block, and f-block:
s-block: This block includes groups 1 and 2, encompassing the alkali metals and alkaline earth metals, respectively. The s-block elements have their outermost electrons in the s orbital. For example, hydrogen and helium (period 1), and lithium and beryllium (period 2) are part of the s-block.
p-block: Encompassing groups 13 to 18, the p-block contains elements that have their outermost electrons in the p orbital. This block includes a diverse range of elements, from metals and metalloids to nonmetals and noble gases, such as nitrogen, oxygen, and neon.
d-block: This block consists of the transition metals, which are found in groups 3 to 12. The d-block elements are characterized by having their outermost electrons in the d orbital. These metals, including iron, copper, and gold, are known for their ability to form various oxidation states and complex compounds.
f-block: The f-block contains the inner transition metals, which are the lanthanides and actinides. These elements have their outermost electrons in the f orbital. The f-block elements are typically shown as a separate section at the bottom of the periodic table to maintain its compact form and include elements like uranium and neodymium.
Periodic Trends
Atomic Size
As you move across a period in the periodic table, from left to right, the atomic size generally decreases. This decrease is due to the increasing nuclear charge, which results from a greater number of protons in the nucleus of each successive element. The additional protons exert a stronger attractive force on the electron cloud, pulling the electrons closer to the nucleus.
Despite the increasing number of electrons across a period, the added electrons enter the same outer shell and do not significantly increase the shielding effect between the outer electrons and the nucleus. As a result, the effective nuclear charge (the net positive charge experienced by the outermost electrons) increases, causing the electron cloud to be drawn tighter and closer to the nucleus. This effect leads to a decrease in the atomic radius or size as you proceed across a period.
Ionization Energy
Ionization energy, which is the energy needed to remove an electron from an atom, generally rises as you move across a period from left to right in the periodic table. This increase is because the nuclear attraction for the electrons becomes stronger with each successive element. As the number of protons in the nucleus increases, the positive charge of the nucleus grows, enhancing its ability to hold onto the electrons more tightly.
This stronger nuclear attraction makes it more difficult to remove an electron, thereby raising the ionization energy. Consequently, elements toward the end of a period, such as the noble gases, have higher ionization energies because their outer electrons are more strongly bound to the nucleus. This trend in ionization energy is a key factor in determining the chemical reactivity of elements, with elements having higher ionization energies generally being less reactive.
Electronegativity
Electronegativity refers to the ability of an atom to attract and hold onto shared electrons when it forms a chemical bond. As you move from left to right across a period on the periodic table, electronegativity generally increases. This is because the atoms have a greater number of protons in their nuclei, leading to a stronger attraction for electrons.
The increase in nuclear charge means that the electrons in a bond are pulled more strongly toward the atom with higher electronegativity. Therefore, elements towards the end of a period, especially the nonmetals, are more electronegative and have a greater tendency to attract electrons in a bond. This trend is crucial for understanding the nature of chemical bonds in different compounds, as the difference in electronegativity between atoms can determine the type of bond (ionic, polar covalent, or nonpolar covalent) that will form.
Related Links
Alkaline Earth Metals
Element Groups
Halogens
Periodic Table