Solute
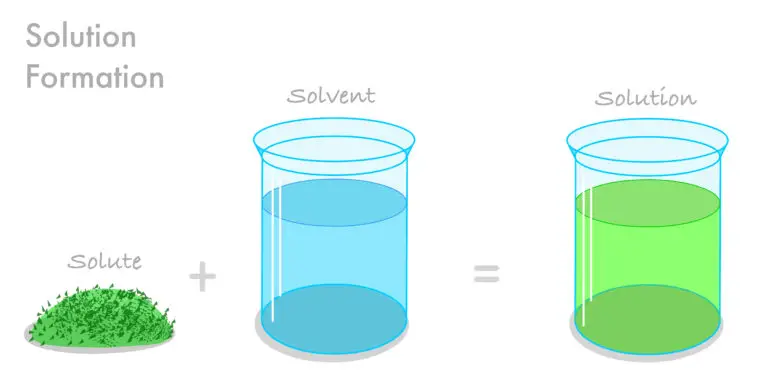
Table of Contents
Solute Definition
A solute is the component of a solution that is dissolved in another substance, known as the solvent. The resulting homogeneous mixture is called a solution. Solutes can exist in different physical states—solid, liquid, or gas—depending on the nature of the solution and the conditions under which it is formed.
For example, in a saltwater solution, the salt is the solute that dissolves in water, the solvent. If the solution is sugar dissolved in water, the sugar acts as the solute. In the air we breathe, which can be considered a solution of gases, oxygen is a solute that is dissolved in nitrogen, the primary solvent.
The state of the solute before it is dissolved can vary: salt and sugar are solid solutes, while oxygen is a gaseous solute. The amount of solute in a solution is typically less than the amount of solvent, but this can vary depending on the concentration of the solution.
Solutes and Solutions
Role in Solutions
Solutes play a key role in creating and defining the properties of solutions. When a solute disperses in a solvent, it breaks down into smaller particles, such as molecules, atoms, or ions, uniformly distributed throughout the solvent, forming a homogeneous mixture or solution. The solute’s presence and nature significantly influence the solution’s properties and behavior.
Color: Certain solutes can impart color to solutions based on their chemical nature and how they interact with light. For example, the copper sulfate solute gives a blue color to its aqueous solution.
Taste: The solute can also affect the taste of a solution; for instance, sugar, when dissolved in water, gives a sweet taste, while salt results in a saline taste.
Odor: Some solutes contribute to the smell of a solution due to their volatile nature, which allows them to evaporate and be detected as an odor.
Conductivity: The electrical conductivity of a solution is often determined by the presence of ionic solutes. When salts dissolve in water, they disassociate into ions that can carry electric current.
Chemical reactivity: The type and concentration of solutes in a solution can significantly affect the reaction rates and chemical equilibrium, influencing how the solution reacts with other substances.
Solubility
The solubility of a solute is a measure of how well it can dissolve in a solvent at given conditions, including temperature, pressure, and the specific chemical interactions between the solute and solvent. This property is fundamental in understanding and predicting how substances will behave when mixed.
Temperature: Solubility can be significantly affected by temperature changes. Generally, the solubility of solid solutes in liquid solvents increases with rising temperature, while the solubility of gases in liquids typically decreases as temperature increases.
Pressure: Pressure primarily influences the solubility of gases in liquids; an increase in pressure can lead to greater solubility of the gas in the liquid, as described by Henry’s law.
Solute-solvent interactions: The chemical nature of both the solute and the solvent plays a critical role in solubility. Polar solutes tend to dissolve better in polar solvents, and nonpolar solutes are more soluble in nonpolar solvents, following the principle that “like dissolves like.”
Solubility is quantitatively expressed in units such as grams per liter (g/L) or moles per liter (mol/L), indicating how much solute can be dissolved in a certain volume of solvent under specified conditions.
Saturation
A solution is considered saturated when it contains the maximum amount of solute that can dissolve in the solvent at specific temperature and pressure conditions. In a saturated solution, the solvent has reached its capacity to dissolve the solute, and the solution is in a dynamic equilibrium where the solute’s dissolution rate equals the precipitation rate, meaning that as many solute particles are dissolving as are crystallizing or precipitating.
If additional solute is added to a saturated solution, it will not dissolve; instead, it may form a precipitate or add to the solid phase at the bottom or surface of the solution. This occurrence is due to the solvent’s inability to accommodate more solute particles in solution, leading to the excess solute separating out as a distinct phase.
Concentration
The concentration of a solute in a solution quantifies the amount of that solute dissolved in a specific volume or mass of solvent, indicating the strength or intensity of the solution. This measurement is crucial in various scientific and industrial applications, as it directly impacts the properties and behaviors of the solution. Concentration can be expressed in different units, depending on the context and specific needs:
Mass/volume (e.g., grams per liter, g/L): This common unit of concentration measures the mass of the solute in a given volume of solution. It is widely used in chemistry, medicine, and environmental studies to express the amount of a substance in a liquid.
Moles/volume (molarity, M): Molarity is a widely used concentration unit in chemistry, defined as the number of moles of solute per liter of solution. It is especially useful in chemical reactions and stoichiometry because it directly relates to the number of molecules or ions in the solution.
Mass/mass (percent concentration): This unit expresses concentration as the mass of the solute divided by the total mass of the solution, multiplied by 100 to give a percentage. It is often used in industrial formulations, pharmacology, and nutrition.
Types of Solutes
Solid Solutes
Solid solutes, such as salt (sodium chloride, NaCl), sugar (sucrose), metals, minerals, and a wide range of organic compounds, can dissolve in solvents to form solutions. When these solid solutes are mixed with liquid solvents like water, alcohol, or acids, they undergo a dissolution process, where the solid particles break down and disperse uniformly throughout the solvent, creating a homogeneous mixture.
Salt (NaCl) dissolves in water to form an electrolyte solution, separating into sodium (Na⁺) and chloride (Cl⁻) ions, which are essential for many biological processes.
Sugar (sucrose) dissolves in water through the interaction of water molecules with sugar molecules, leading to a sweet-tasting solution used in a variety of culinary applications.
Metals can dissolve in certain acidic solutions, forming metal ions and compounds that may be used in industrial processes, like metal plating and purification.
Minerals such as calcium carbonate (CaCO₃) dissolve in water, especially when the water is slightly acidic, affecting geological and environmental processes like rock formation and water hardness.
Organic compounds, which include a vast array of chemicals such as pharmaceuticals, dyes, and flavoring agents, can dissolve in various solvents, influencing their behavior, stability, and interactions in different environments.
Liquid Solutes
Liquid solutes are substances in liquid form that dissolve in other liquids to create solutions. Examples of liquid solutes include ethanol, commonly known as alcohol, which is used in beverages and as a solvent in industries; acetic acid, the main component of vinegar; and glycerol, a sweet-tasting liquid used in food and pharmaceutical products. Organic solvents like benzene, methanol, and acetone also fall into this category.
The solubility of these liquid solutes in various solvents depends on their chemical nature and the principle of “like dissolves like.” For instance, ethanol and water mix well together because both are polar substances, allowing them to interact and form hydrogen bonds. Conversely, nonpolar liquid solutes, such as certain organic solvents, are more soluble in nonpolar solvents but may not dissolve well in polar solvents like water.
Gas Solutes
Liquid solutes are substances in liquid form that dissolve in other liquids to create solutions. Examples of liquid solutes include ethanol, commonly known as alcohol, used in beverages and as a solvent in industries; acetic acid, the main component of vinegar; and glycerol, a sweet-tasting liquid used in food and pharmaceutical products. Organic solvents like benzene, methanol, and acetone also fall into this category.
The solubility of these liquid solutes in various solvents depends on their chemical nature and the principle of “like dissolves like.” For instance, ethanol and water mix well because both are polar substances, allowing them to interact and form hydrogen bonds. Conversely, nonpolar liquid solutes, such as certain organic solvents, are more soluble in nonpolar solvents but may not dissolve well in polar solvents like water.
Related Links
Chemical Bond
Chemical Reaction
Exothermic Reaction
Redox Reaction