Antibody
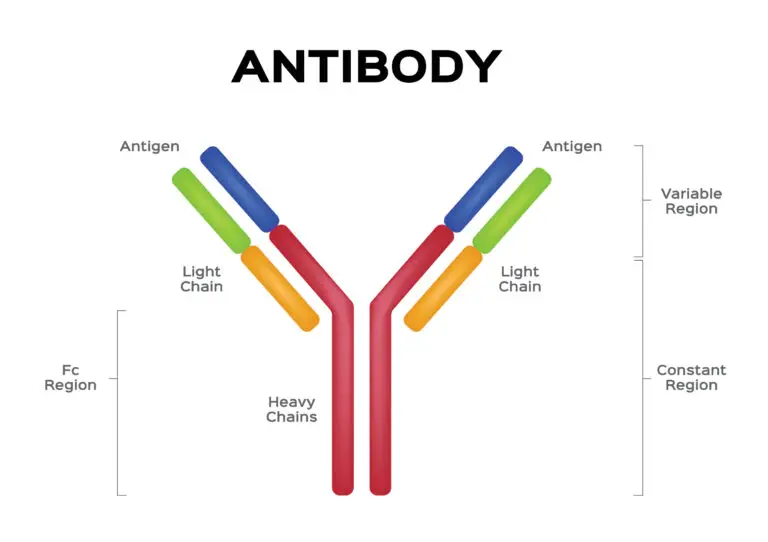
Table of Contents
What is an Antibody?
An antibody, also known as an immunoglobulin, is a large Y-shaped protein produced by the immune system in response to the presence of foreign substances called antigens. Antibodies play a crucial role in the immune system’s ability to recognize and neutralize harmful pathogens, such as bacteria, viruses, and other microorganisms.
Key Features of Antibodies
Structure
An antibody molecule comprises four polypeptide chains: two identical heavy (H) chains and two identical light (L) chains. Disulfide bonds link together these chains to form a flexible Y-shaped molecule.
The shape of the antibody facilitates its function in the immune response. The stem of the Y, known as the Fc (Fragment crystallizable) region, interacts with immune cells and other molecules in the immune system. The arms of the Y, known as the Fab (Fragment antigen-binding) regions, contain sites that bind specifically to antigens.
Each antibody has a variable region and a constant region. The variable region, located at the tips of the Y-shaped structure, is responsible for the antibody’s antigen-binding specificity. This region varies greatly among antibodies, allowing the immune system to recognize various antigens. The constant region in the rest of the molecule determines the antibody’s class and role in the immune response.
Antigen Binding
The antibody’s variable region contains unique amino acid sequences that form a specific three-dimensional structure. This structure can bind with high specificity to a complementary structure on the antigen, much like a lock and key. This binding mechanism allows antibodies to target and neutralize specific pathogens or infected cells effectively.
Antibody Classes
Antibodies, or immunoglobulins, are classified into several classes based on differences in their constant region, particularly in the heavy chain structure. Each class of antibody, designated by the letters IgA, IgD, IgE, IgG, and IgM, has distinct roles and characteristics within the immune system:
IgG: This is the most abundant type of antibody in the body and is crucial for long-term immunity. IgG antibodies are the main type found in blood and extracellular fluid, allowing them to control infection of body tissues. They provide protection against bacteria, viruses, and can trigger other immune cells to destroy foreign substances. IgG can cross the placenta and provide passive immunity to the fetus.
IgM: This is the first class of antibody the body makes when it fights a new infection. IgM molecules are large, primarily staying in the bloodstream, where they are very effective at killing bacteria and initiating an immune response. Their presence often indicates a current or recent infection.
IgA: This antibody is found in high concentrations in the mucous membranes, particularly those lining the respiratory and digestive tracts, as well as in saliva, tears, and breast milk. IgA plays a crucial role in the immune function of mucous membranes by protecting against infections at these barriers.
IgE: These antibodies are found in the lungs, skin, and mucous membranes. IgE is best known for its role in allergic reactions; it triggers the release of histamine, which causes the symptoms of an allergy. IgE also plays a role in protection against parasitic worms.
IgD: This class of antibodies is present in small amounts in the blood and is primarily found on the surface of immature B-cells, which are immune cells that produce antibodies. IgD’s role is not completely understood, but it is believed to be involved in initiating immune responses.
Production
Antibodies are produced by B cells (B lymphocytes), a type of white blood cell. When a B cell encounters an antigen that matches its specific antibody, it undergoes activation and differentiation into plasma cells, which release large quantities of antibodies.
Functions
- Neutralization: Antibodies can neutralize pathogens by binding to them and preventing them from infecting host cells.
- Opsonization: Antibodies can tag pathogens for destruction by immune cells through phagocytosis.
- Complement Activation: Antibodies can activate the complement system, a group of proteins that enhance the immune response.
Memory and Immune Memory
Memory B cells are formed during an immune response, providing long-term immunity. Upon re-exposure to the same antigen, memory B cells can quickly produce large quantities of antibodies to mount a faster and more effective immune response.
Primary and Secondary Immune Responses
The first encounter with an antigen triggers a primary immune response, producing specific antibodies. Upon subsequent exposures, the secondary immune response is faster and more robust due to the presence of memory B cells.
Monoclonal Antibodies
Monoclonal antibodies are laboratory-produced antibodies designed for specific therapeutic, diagnostic, or research purposes. They are produced from a single B cell type or a hybridoma cell line.
Related Links
The Immune System
What are Pathogens
Bacteria
Microorganisms