Atom
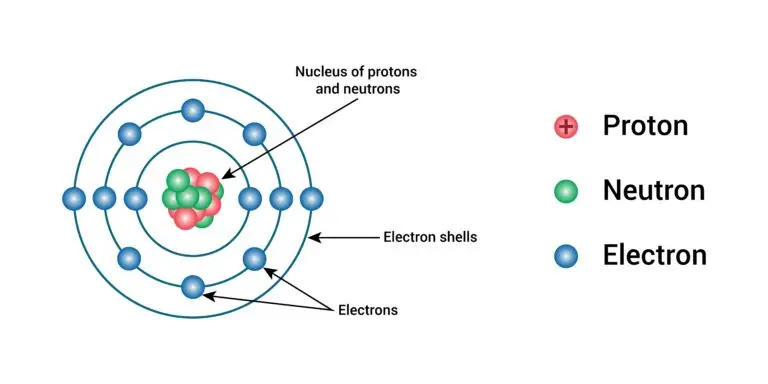
Table of Contents
What is an Atom
An atom is the smallest unit of an element that retains the chemical properties of that element. It consists of a nucleus containing protons and neutrons, surrounded by electrons orbiting the nucleus.
An atom’s protons, neutrons, and electrons arrangement contributes to its overall structure and properties. The number of protons in the nucleus determines the element’s atomic number, while the number of protons and neutrons in the nucleus gives the atom’s atomic mass.
The electrons in an atom are arranged in orbitals according to specific rules, such as the Aufbau principle and the Pauli exclusion principle, which govern electron configurations and the stability of atoms.
Properties of Atoms
Nucleus
The nucleus is like the “heart” of an atom, located at its center. It comprises two types of particles: protons, which have a positive charge (+1), and neutrons, which don’t have any charge (they’re neutral).
Protons are like the “superheroes” of the nucleus, carrying a positive charge and playing a crucial role in defining the atom’s identity. The number of protons in the nucleus gives each element its unique atomic number.
This atomic number is like the atom’s “ID number” and helps scientists identify and categorize different elements. So, in a nutshell, the nucleus is where all the action happens in an atom, with protons and neutrons working together to determine what kind of element it is.
Electrons
Electrons are tiny particles with a negative charge (-1) that zoom around the nucleus of an atom. They don’t just fly randomly; they follow specific paths called energy levels or electron shells. These shells are like “orbitals” for the electrons, arranged in increasing energy levels. The innermost shell is closest to the nucleus and has the lowest energy, while the outermost shell is farther away and has higher energy levels.
Now, here’s where it gets interesting: the outermost shell, also called the valence shell, is like the “personality” of the atom. It’s what determines how the atom behaves chemically and how reactive it is. The number of electrons in this valence shell and how easily they can be shared or transferred with other atoms play a big role in chemical reactions.
For example, atoms with a full valence shell are stable and less likely to react, while atoms with incomplete valence shells are more reactive and eager to bond with other atoms to achieve stability. So, the valence shell is like the “social circle” of the atom, influencing its interactions and chemical properties.
Atomic Structure
The atomic structure of an atom is often represented symbolically using the element’s atomic symbol, atomic number (which is the number of protons in the nucleus), and mass number (which is the sum of protons and neutrons in the nucleus).
For instance, the symbol for carbon is “C,” indicating that it is the element carbon. Its atomic number is 6, which tells us that a carbon atom has 6 protons in its nucleus.
The most common isotope of carbon, which is Carbon-12 (written as 12C), has a mass number of 12. This mass number is the total number of protons and neutrons in the nucleus. Since carbon has 6 protons (as indicated by its atomic number), Carbon-12 must have 6 neutrons (12 – 6 = 6). Therefore, the atomic structure of Carbon-12 can be represented as “C-12,” where “C” is the symbol for carbon, and “12” represents its mass number.
Isotopes
Isotopes are basically versions of the same element with a different number of neutrons in their nuclei. Despite having the same number of protons (which defines the element’s identity), isotopes can vary in their mass numbers due to the differing neutron counts. However, because they share the same number of protons, isotopes are chemically identical. This means they behave the same way in chemical reactions and have the same chemical properties.
For example, carbon has three isotopes: Carbon-12 (12C), Carbon-13 (13C), and Carbon-14 (14C). They all have 6 protons (since carbon’s atomic number is 6), but their neutron counts differ: Carbon-12 has 6 neutrons, Carbon-13 has 7 neutrons, and Carbon-14 has 8 neutrons. Even though these isotopes may have slightly different physical properties due to their varying masses, their chemical behavior remains the same.
Subatomic Particles
Atoms are made up of subatomic particles: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, which is at the center of the atom. On the other hand, electrons are found in orbitals or electron shells surrounding the nucleus.
The protons and neutrons in the nucleus primarily determine the mass of an atom. Protons have a positive charge and a relatively large mass, while neutrons have no charge (they are neutral) but have a similar mass to protons. Collectively, protons and neutrons contribute most of the mass of an atom.
In contrast, electrons have a very small mass compared to protons and neutrons. Their contribution to the overall mass of an atom is negligible. Despite their small mass, electrons play a crucial role in determining the chemical properties of an atom, including how it interacts with other atoms to form compounds and molecules.
The mass of an atom is primarily due to the protons and neutrons in the nucleus. At the same time, electrons contribute very little to the overall mass but are important for understanding the atom’s behavior in chemical reactions.
Electron Configuration
The arrangement of electrons in an atom’s electron shells is called its electron configuration. This configuration describes how electrons are distributed among the various energy levels, shells, and subshells around the atom’s nucleus.
Electrons fill shells and subshells following specific rules:
Aufbau Principle: Electrons first occupy the lowest energy levels (shells) before filling higher energy levels. This principle helps determine the order in which electrons fill the orbitals within an atom.
Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins. This means that if two electrons occupy the same orbital, they must have opposite spins (one with spin up and the other with spin down).
Hund’s Rule: When filling degenerate (equal-energy) orbitals (such as p orbitals), electrons prefer to occupy empty orbitals first before pairing up. This leads to the arrangement of electrons with parallel spins in different orbitals of the same energy level.
The electron configuration of an atom is crucial in determining its chemical properties and bonding behavior. It provides information about the distribution of electrons in the atom’s orbitals, which influences how the atom interacts with other atoms to form chemical bonds. Understanding electron configuration helps predict an atom’s reactivity, stability, and the types of chemical bonds it can form.
Atomic Size
The size of an atom depends on how far the outermost electron shell is from the nucleus. Imagine the nucleus as the “center” of the atom and the electron shell as a sort of “orbit” around it. When we look at the periodic table, we notice that as we move down a group (a column) in the table, atoms generally get bigger. This happens because atoms in the same group have more electron shells as you go down, like adding layers to a balloon, which makes the atom larger overall.
On the other hand, when we move across a period (a row) in the periodic table, atomic size tends to decrease from left to right. This is because the number of protons in the nucleus increases as you move across the period, creating a stronger “pull” on the electrons. It’s like having a stronger magnet in the center that attracts the electrons closer, making the atom smaller overall.
The distance between the nucleus and the outermost electron shell determines atomic size. Moving down a group increases atomic size due to added electron shells. Moving across a period decreases atomic size because the stronger nuclear charge pulls electrons closer to the nucleus.
Chemical Bonding
Atoms interact through various types of chemical bonding, leading to the formation of molecules and compounds. These bonds are crucial for stabilizing atoms and creating new substances with unique properties. The main types of chemical bonding include:
Covalent Bonding: In covalent bonding, atoms share electrons to achieve a stable electron configuration. This type of bonding commonly occurs between nonmetal atoms. Each atom contributes one or more electrons to form a shared pair or multiple pairs of electrons. Covalent bonds are strong and can form single, double, or triple bonds depending on the number of shared electrons.
Ionic Bonding: Ionic bonding involves the transfer of electrons from one atom to another, forming positively charged ions (cations) and negatively charged ions (anions). This type of bonding typically occurs between a metal atom (which loses electrons to become a cation) and a nonmetal atom (which gains electrons to become an anion). The attraction between oppositely charged ions creates an ionic bond.
Weak Forces:
- Van der Waals Forces: These are weak, attractive forces between molecules or atoms due to temporary fluctuations in electron distribution. Van der Waals forces include London dispersion forces (arising from temporary dipoles) and dipole-dipole interactions (between permanent dipoles).
- Hydrogen Bonding: Hydrogen bonding occurs when a hydrogen atom covalently bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) interacts with another electronegative atom. This results in a strong dipole-dipole attraction, commonly seen in molecules like water and DNA.
Related Links
Cells
Nucleus
Proton
The Cell Cycle