Atomic Number
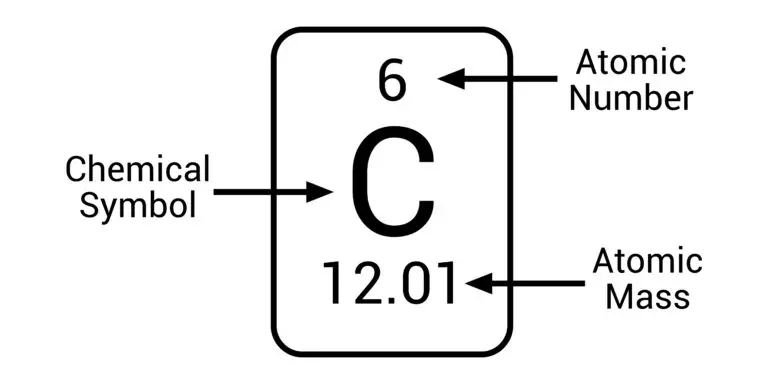
Table of Contents
Atomic Number (Periodic Table)
The atomic number of an element is like its “identity badge” in the world of atoms. It tells us the number of protons hanging out in the nucleus of an atom belonging to that specific element.
Now, because each element has a unique number of protons, the atomic number serves as a sort of “name tag” that distinguishes one element from another.
For example, hydrogen’s atomic number is 1, which means it has one proton in its nucleus. On the other hand, carbon has an atomic number of 6 because it has six protons.
Identifying an Element
Protons
Protons are like the “cheerful” team members found in the nucleus of an atom. They are positively charged subatomic particles, each carrying a charge of +1. Imagine them as tiny magnets with a positive end! These protons play a crucial role in defining the identity of an element because the number of protons in the nucleus determines the element’s atomic number.
For instance, let’s take hydrogen. A hydrogen atom has just one proton in its nucleus, which gives it an atomic number of 1. This means that you know you’re dealing with hydrogen whenever you see an atomic number of 1. Similarly, carbon has six protons in its nucleus, giving it an atomic number of 6. So, whenever you see an atomic number of 6, you know it’s carbon.
Element Identification
The atomic number is like the “address” that helps us find elements in the periodic table. It’s a fundamental characteristic because each element has a unique atomic number, which is simply the number of protons in its nucleus. This number is crucial because it tells us exactly what element we’re dealing with.
When you look at the periodic table, you’ll notice that elements are arranged in a specific order. This order is based on increasing atomic number. It starts with hydrogen, which has the atomic number 1 because it has one proton in its nucleus. Then comes helium with an atomic number of 2, lithium with 3, and so on, following this sequence of increasing atomic numbers.
This organization is super helpful because it tells us the name of the element and important information about its properties and behavior. For example, elements with similar atomic numbers tend to have similar properties and behave similarly. So, by knowing the atomic number, we can quickly identify an element and learn a lot about it just by looking at its place in the periodic table. And as of the latest periodic table, we’ve discovered elements up to oganesson, with an atomic number of 118, making it the heaviest element we know of so far!
Electron Configuration
The atomic number not only helps identify elements but also plays a key role in determining the number of electrons in a neutral atom of the element. In a neutral atom, the number of electrons equals the number of protons, creating a balance between positive and negative charges.
Here’s how it works: since protons have a positive charge and electrons have a negative charge, the number of electrons in an atom matches the number of protons in its nucleus. This equal number of positive and negative charges ensures the atom is electrically neutral overall. For example, hydrogen, which has an atomic number of 1, has one proton in its nucleus and one electron orbiting around it, maintaining electrical neutrality.
This balance of charges is crucial for the stability of atoms. If an atom were to gain or lose electrons, it would become an ion with a net positive or negative charge respectively. However, in its neutral state, the number of electrons precisely matches the atomic number (number of protons), resulting in a balanced atom with no overall charge.
Isotopes and Atomic Number
Isotopes of an element are like “versions” of that element with different weights, but they still share the same “identity number”.
Here’s where isotopes get interesting: even though they have the same atomic number (because they’re all the same element), they can have different mass numbers. This difference in mass numbers is because isotopes have varying numbers of neutrons in their nuclei. Neutrons don’t carry any charge like protons but add weight to the atom.
Because isotopes have the same number of protons and electrons, they exhibit similar chemical behaviors. This means they can bond with other atoms similarly and form the same types of compounds. However, because they have different numbers of neutrons, isotopes may have different physical properties. For example, some isotopes might be heavier or more stable than others.
In essence, isotopes are like siblings with the same name (atomic number) but different ages (mass numbers) and maybe slightly different personalities (physical properties). Understanding isotopes is crucial in fields like chemistry, archaeology (carbon dating), and nuclear physics, where these subtle differences play a significant role.
Periodic Trends
The atomic number is like a “map key” that helps us navigate the periodic table and understand its trends. As we move across a period (a row) from left to right, the atomic number increases, and this sequential increase influences various properties of the elements.
Firstly, let’s talk about atomic size. As you move from left to right across a period, the atomic size generally decreases. This happens because the increasing atomic number corresponds to an increase in the number of protons in the nucleus. These extra protons exert a stronger positive charge on the electrons, pulling them closer to the nucleus and making the atom smaller overall.
Next, let’s consider ionization energy. Ionization energy is the energy required to remove an electron from an atom. As the atomic number increases across a period, the ionization energy generally increases as well. This is because the higher positive charge in the nucleus (due to more protons) holds the electrons more tightly, making it harder to remove them.
Finally, let’s discuss electronegativity. Electronegativity is a measure of an atom’s ability to attract and hold onto electrons in a chemical bond. Similar to ionization energy, electronegativity tends to increase as you move across a period from left to right. The increasing atomic number correlates with a stronger attraction for electrons, making elements more electronegative as you move towards the right side of the periodic table.
Molar Mass
The atomic number isn’t just about identifying elements; it also connects to something called molar mass, like the “weight” of an element in a specific amount called a mole. Now, the molar mass of an element is roughly equal to its atomic mass, which is based on adding up the protons and neutrons in the nucleus.
Here’s how the atomic number comes into play with molar mass: since the atomic mass (which contributes to molar mass) includes the number of protons and neutrons in the nucleus, the atomic number indirectly influences the molar mass of an element. This is because more protons and neutrons mean a higher atomic mass and, thus, a higher molar mass.
For example, let’s take helium and carbon. Helium has an atomic number of 2 and a relatively low molar mass because it has only a few protons and neutrons. On the other hand, carbon has an atomic number of 6 and a higher molar mass because it has more protons and neutrons in its nucleus.
In a way, the atomic number gives us a clue about an element’s molar mass. Elements with higher atomic numbers tend to have higher molar masses because more particles (protons and neutrons) are packed into their nuclei.
Atomic Number Lookup
Instructions: Enter the atomic number of an element from the periodic table to see its information.
Related Links
Elements
Periodic Table
Isotopes
Mass Number