Catalyst (Chemistry)
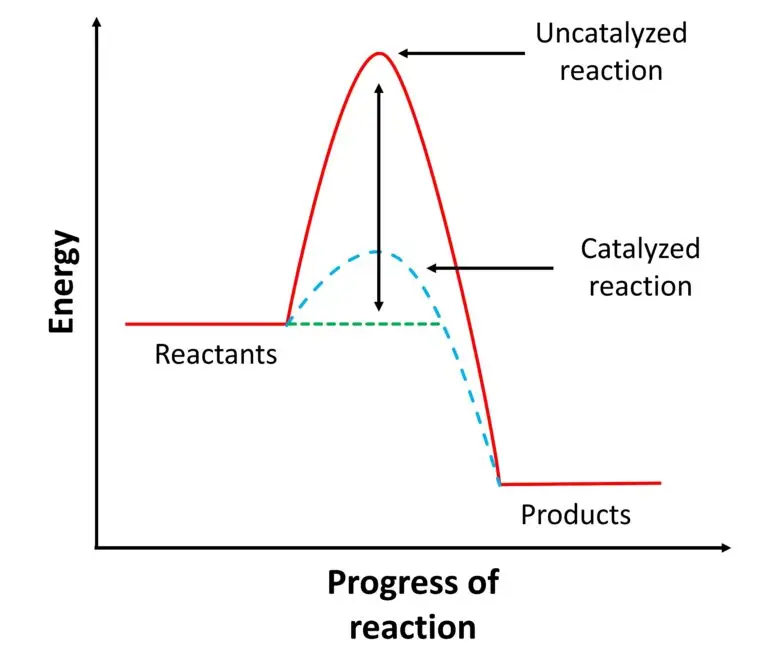
Table of Contents
What is a Catalyst
A catalyst is like a “helper” in chemistry that makes reactions happen faster without getting used up. It creates a shortcut for the reaction to follow, which requires less energy to get started. This shortcut is called an alternative pathway, and the energy needed to kickstart the reaction on this pathway is called activation energy.
Imagine you’re trying to climb a hill. Usually, climbing the steep hill would take a lot of effort, representing the high activation energy needed for a reaction to start without a catalyst. But if you had a smooth path around the hill, reaching the other side would be much easier and faster. That’s what a catalyst does—it provides this smoother path, lowering the activation energy required for the reaction to occur.
The important thing to note about catalysts is that they don’t get used up in the reaction. They can be used repeatedly to speed up the same reaction multiple times. This makes catalysts incredibly valuable in many industries and processes where speeding up reactions can save time, energy, and resources.
Catalyst "Helper"
Activation Energy
Catalysts are like helpers in chemistry that make reactions happen faster by reducing the amount of energy needed to start the reaction. This energy, called activation energy, is the minimum energy required for reactant molecules to transform into products during a chemical reaction. Catalysts lower this energy barrier, making it easier for the reaction to proceed and allowing it to occur at a faster rate.
To understand this concept better, imagine a ball rolling uphill. The higher the hill, the more energy it needs to reach the top. Similarly, in a chemical reaction, reactant molecules need a certain amount of energy to “climb” the energy hill and transform into products. This energy hill represents the activation energy required for the reaction to occur.
Now, when a catalyst is added to the reaction, it creates a sort of “shortcut” or a smoother path for the reactant molecules. This shortcut reduces the height of the energy hill, making it easier for the molecules to reach the top and form products. As a result, the reaction can proceed faster because less energy is needed for the molecules to react.
Types of Catalysts
Catalysts can be categorized into two main types: homogeneous catalysts and heterogeneous catalysts.
Homogeneous catalysts are in the same phase (state) as the reactants they are working with. For example, if a catalyst is dissolved in a liquid reaction mixture and interacts with other substances also in the liquid phase, it is considered a homogeneous catalyst. This type of catalyst remains uniformly distributed throughout the reaction mixture.
On the other hand, heterogeneous catalysts are in a different phase compared to the reactants. For instance, if a catalyst is in a solid form and interacts with gaseous or liquid reactants, it is classified as a heterogeneous catalyst. Unlike homogeneous catalysts, heterogeneous catalysts exist as separate entities from the reactants and may be present as solids, liquids, or gases while the reaction occurs.
To illustrate, consider a chemical reaction where a metal catalyst is suspended in a liquid solution of reactants. Here, the metal catalyst and the reactants are in the same phase (liquid), making it a homogeneous catalytic system.
In contrast, if the same metal catalyst is solid and interacts with gaseous reactants, it would be considered a heterogeneous catalyst due to the phase difference between the catalyst and the reactants.
Reaction Mechanism
Catalysts facilitate chemical reactions by offering an alternative pathway with a lower activation energy compared to the uncatalyzed reaction. This alternative pathway often involves the formation of an intermediate complex between the catalyst and the reactant molecules, leading to easier product formation.
Here’s how it works: In an uncatalyzed reaction, reactant molecules need to overcome a certain energy barrier (activation energy) to transform into products. This barrier can be quite high, making the reaction slow and inefficient. However, when a catalyst is introduced, it interacts with the reactant molecules to form an intermediate complex. This complex is more stable and has a lower energy requirement to proceed to the products.
The catalyst essentially provides a “shortcut” for the reaction, allowing it to occur more readily and at a faster rate. This is because the intermediate complex has a lower activation energy, making it easier for the reactant molecules to transition into products. After the reaction is complete, the catalyst is regenerated and can be used again in subsequent reactions, as it is not consumed during the reaction.
Reusability
Catalysts are incredibly valuable in chemical processes because they are not consumed in catalyzing reactions. This unique property allows catalysts to be used repeatedly, making them cost-effective and environmentally friendly.
When a catalyst facilitates a reaction, it undergoes temporary interactions with the reactant molecules to lower the activation energy and speed up the reaction. However, once the reaction is complete, the catalyst remains unchanged and can be recovered from the reaction mixture. This means that the catalyst is not used up or altered during the reaction, making it available for use in subsequent reactions.
The reusability of catalysts has several essential benefits. Firstly, it reduces the overall cost of chemical processes, as the same catalyst can be employed multiple times without requiring frequent replacement. This can lead to significant savings in terms of resources and production costs. Additionally, the reusability of catalysts contributes to environmental sustainability by reducing waste generation. Since the catalyst is not consumed, less waste is produced from the reaction, resulting in a cleaner and more efficient chemical production process.
Effect on Equilibrium
Catalysts play a unique role in chemical reactions by accelerating the rates of both the forward and reverse reactions equally. However, it’s important to note that catalysts do not affect the equilibrium position of a reaction. This means that while catalysts speed up the attainment of equilibrium, they do not alter the concentrations of reactants or products once equilibrium is reached.
In a reversible reaction, such as A + B ⇌ C + D, the reaction can proceed in both the forward (A + B to C + D) and reverse (C + D to A + B) directions. At equilibrium, the rates of the forward and reverse reactions are equal, and the concentrations of reactants and products remain constant over time.
When a catalyst is introduced into this system, it speeds up both the forward and reverse reactions. This acceleration allows the reaction to reach equilibrium faster than without a catalyst. However, once equilibrium is achieved, the concentrations of reactants and products are determined solely by the equilibrium constant (K), and the presence of a catalyst does not change these concentrations.
Temperature and Catalysts
Catalysts influence the temperature at which a reaction occurs efficiently. Based on their temperature preferences, there are two main types of catalysts: thermocatalysts and cold catalysts.
Thermocatalysts: These catalysts are more effective at higher temperatures. They work efficiently in reactions that require elevated temperatures to proceed at a desirable rate. Thermocatalysts help overcome the activation energy barrier at higher temperatures, leading to faster reaction rates and improved efficiency. Examples of thermocatalysts include specific metal catalysts used in high-temperature processes like industrial chemical reactions and combustion reactions.
Cold Catalysts: Conversely, cold catalysts are effective at lower temperatures. They can facilitate reactions that occur efficiently at room or slightly elevated temperatures. Cold catalysts benefit reactions where high temperatures may not be desirable due to energy costs, safety concerns, or product stability considerations. These catalysts provide a lower-energy pathway for reactions at lower temperatures, contributing to energy savings and process optimization. Cold catalysts find applications in various fields, including pharmaceuticals, food processing, and environmental remediation.
The choice between thermocatalysts and cold catalysts depends on the specific reaction requirements, desired reaction rates, energy considerations, and product stability concerns.
Catalyst Examples
Examples of catalysts span across various domains, showcasing their versatility and importance in different applications:
Enzymes: Enzymes are biological catalysts found in living organisms. They play crucial roles in biochemical reactions by accelerating reactions without being consumed. Examples include digestive enzymes like amylase, protease, and lipase, which help break down food molecules in the digestive system.
Industrial Catalysts: Industrial catalysts are used in large-scale chemical processes. Platinum, along with palladium and rhodium, is used in catalytic converters in vehicles to convert harmful gases like carbon monoxide (CO), nitrogen oxides (NOx), and hydrocarbons (HC) into less harmful substances like carbon dioxide (CO2), nitrogen (N2), and water (H2O).
Transition Metal Complexes: Transition metal complexes, such as those containing nickel, iron, or cobalt, are widely used as catalysts in organic synthesis and industrial processes. They can catalyze various reactions like hydrogenation, oxidation, and polymerization.
Zeolites: Zeolites are crystalline aluminosilicate minerals with porous structures. They are used as catalysts and adsorbents in petrochemical and environmental applications. Zeolite catalysts are employed in processes like cracking, isomerization, and selective adsorption of molecules.
Acid-Base Catalysts: Acid-base catalysts facilitate reactions by either donating or accepting protons. Examples include sulfuric acid (H2SO4) and sodium hydroxide (NaOH) as acid and base catalysts, respectively, in organic synthesis and chemical transformations.
Related Links
Periodic Table
pH
Reactant
Redox Reaction