Chemical Bond
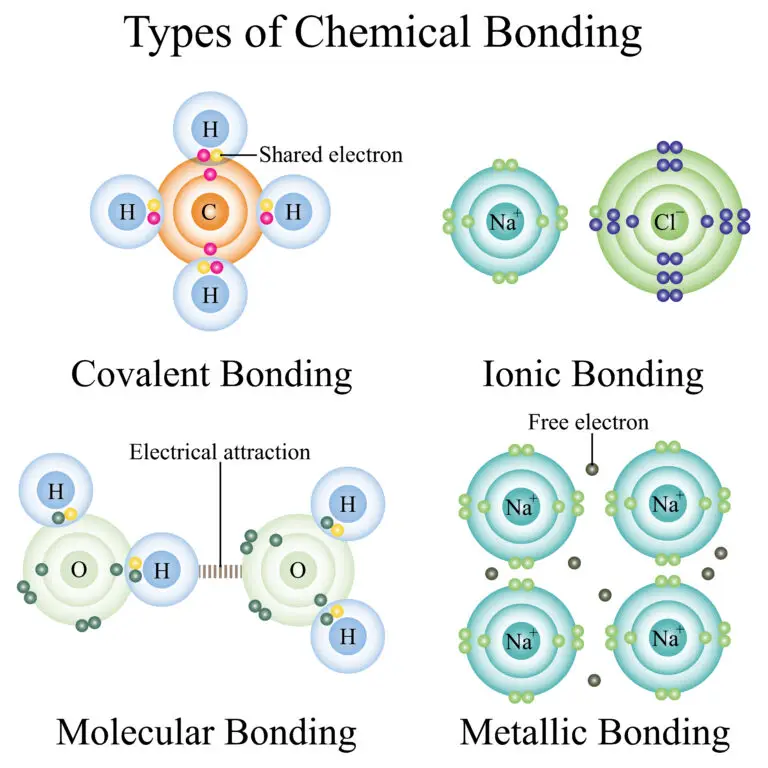
Table of Contents
What are Chemical Bonds
A chemical bond is the attractive force that holds atoms together in molecules or compounds. These bonds are formed by sharing, transferring, or donating electrons between atoms. Imagine atoms as tiny puzzle pieces and the chemical bond as the connection that locks these pieces together to create a larger structure.
How Chemical Bonds Work
Covalent Bonding
Covalent bonds are like a sharing agreement between atoms. They form when atoms share one or more pairs of electrons so that each atom can have a full set of electrons, making them stable and happy. This type of bonding is quite common among nonmetal atoms like oxygen, carbon, and nitrogen.
A classic example of a covalent bond is found in water (H2O). In a water molecule, the oxygen atom shares its electrons with hydrogen atoms, creating a strong bond that holds the molecule together. This sharing of electrons ensures that both the oxygen and hydrogen atoms have a complete set of electrons, making the water molecule stable and able to exist in its liquid form, which we use daily.
Ionic Bonding
Ionic bonds form when atoms play a game of electron transfer. One atom, usually a metal, gives away electrons, becoming a positively charged ion called a cation. The other atom, often a nonmetal, receives these electrons, becoming a negatively charged ion called an anion. These oppositely charged ions are like magnets that attract each other, creating a strong bond known as an ionic bond.
A classic example of an ionic bond is found in sodium chloride (NaCl), which is a common table salt. In NaCl, sodium (Na+) happily donates an electron to chlorine (Cl-), making Na+ positively charged and Cl- negatively charged. These charged ions stick together due to their opposite charges, forming the familiar salt crystals we use in our food.
Metallic Bonding
Metallic bonds are like a dance party among metal atoms. In this bonding, electrons move freely and are not tied to specific atoms. This creates a kind of electron sea that flows through the metal’s structure, holding the metal ions together.
This unique bonding style gives metals special qualities, like conducting electricity and being easily shaped or hammered into different forms (malleability). This free movement of electrons allows metals to pass along electricity and heat so well, making them essential for many everyday objects like wires and cooking pots.
Hydrogen Bonding
Hydrogen bonding is like a friendly handshake between molecules. In one molecule, a hydrogen atom is connected to a very electronegative atom, like oxygen, nitrogen, or fluorine. This electronegative atom pulls the hydrogen atom’s electron cloud closer to itself, creating a partial positive charge on the hydrogen atom.
Now, this partial positive charge on the hydrogen attracts the negative charge of another electronegative atom in a nearby molecule. This attraction is what we call a hydrogen bond. It’s not as strong as a covalent or ionic bond, but it’s strong enough to influence many important properties of substances.
For instance, in water (H2O), hydrogen bonding is why water molecules stick together, creating surface tension and allowing water to form droplets. In DNA, hydrogen bonds between base pairs hold the double helix structure together. Overall, hydrogen bonding plays a crucial role in the behavior of many natural substances.
Van der Waals Forces
Van der Waals forces are like gentle hugs between molecules. They are weak intermolecular forces that occur because of momentary changes in how electrons are distributed within molecules. These forces encompass three types: London dispersion forces, dipole-dipole interactions, and hydrogen bonding.
London Dispersion Forces: These forces are the weakest of the van der Waals forces. They arise from temporary electron density fluctuations, creating temporary molecule dipoles. These temporary dipoles can attract nearby molecules, contributing to interactions between molecules.
Dipole-Dipole Interactions: When polar molecules with permanent dipoles (regions of partial positive and negative charges) interact, they experience dipole-dipole interactions. The positive end of one molecule attracts the negative end of another molecule, leading to a weak attraction between them.
Hydrogen Bonding: Although hydrogen bonding is a van der Waals force type, it’s stronger than London dispersion forces and dipole-dipole interactions. It occurs when a hydrogen atom bonded to an electronegative atom (like oxygen or nitrogen) in one molecule interacts with another electronegative atom in a neighboring molecule. This type of bonding is crucial in biological molecules like DNA and proteins.
Strength of Bonds
The strength of a chemical bond depends on several factors:
Types of Atoms: The atoms involved in bonding play a significant role. For example, bonds between atoms of the same element (like H-H in hydrogen gas) are generally weaker than bonds between different elements (like H-O in water).
Number of Shared or Transferred Electrons: Covalent bonds, where atoms share electrons, are typically stronger than ionic bonds, where electrons are transferred. This is because the shared electrons tie atoms more closely together in covalent bonds.
Distance Between Atoms: The distance between bonded atoms also affects bond strength. Generally, shorter bonds are stronger because the nuclei of the atoms are closer together, leading to more vital attractive forces between them.
In terms of strength ranking:
- Covalent Bonds: Generally the strongest type of bond, especially when atoms share multiple pairs of electrons (double or triple bonds).
- Ionic Bonds: Weaker than covalent bonds because they result from the electrostatic attraction between oppositely charged ions. These bonds can sometimes be strong but are often weaker than covalent bonds.
- Metallic Bonds: Weakest among the three types mentioned. Metallic bonds involve a “sea” of delocalized electrons between metal atoms, leading to weaker attraction between the atoms than covalent or ionic bonds.
Bond Length and Bond Energy
Bond length and bond energy are crucial aspects of understanding the strength of chemical bonds:
Bond Length: This refers to the distance between the nuclei of two bonded atoms. It’s measured in picometers (pm) or angstroms (Å). Generally, shorter bond lengths indicate stronger bonds because the nuclei of the atoms are closer together, leading to stronger attraction between them. In contrast, longer bond lengths suggest weaker bonds with lesser attraction between the atoms.
Bond Energy (Bond Strength): This is the energy required to break a bond and separate the bonded atoms. It’s typically measured in kilojoules per mole (kJ/mol) or electronvolts (eV). Higher bond energies indicate stronger bonds because more energy is needed to break them. Bonds with higher bond energies are more stable and require more energy input to disrupt their structure.
The relationship between bond length and bond energy is straightforward: shorter bond lengths correspond to higher bond energies, indicating stronger bonds with greater attraction between atoms. Conversely, longer bond lengths indicate weaker bonds with lesser attraction and lower bond energies.
Related Links
Chemical Compound
Endothermic Reaction
Energy
Reactant