Diffusion
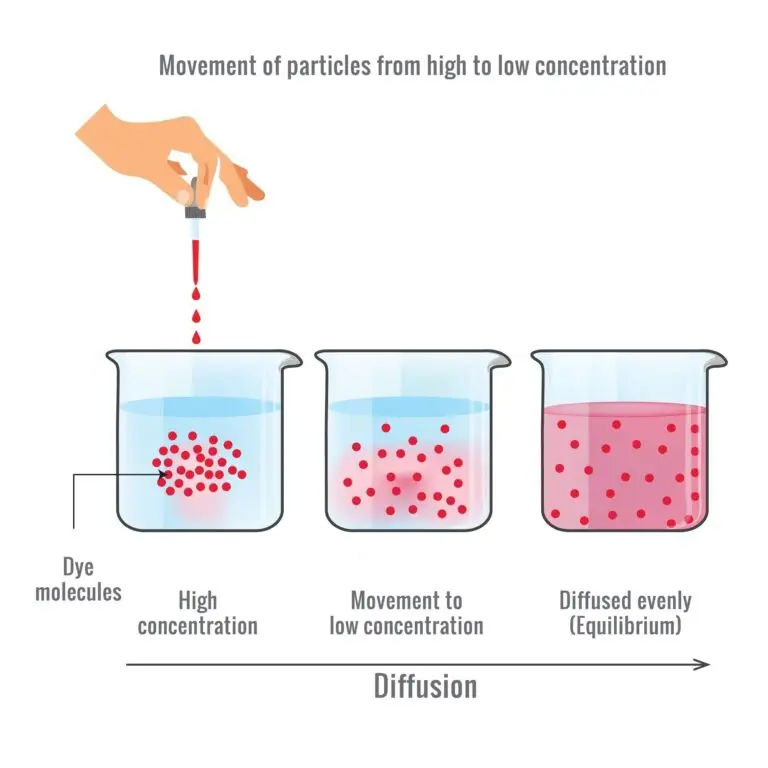
Table of Contents
What is Diffusion?
Diffusion refers to the spontaneous movement of particles (molecules or ions) from an area of higher concentration to an area of lower concentration, driven by the random motion of particles.
This process occurs in various biological systems and is essential for moving substances within and between cells and cellular membranes. Diffusion does not require external energy input and continues until equilibrium is reached.
Diffusion in Physics
Concentration Gradient
- Diffusion occurs along a concentration gradient, which is the difference in the concentration of a substance between two regions.
- Particles move from areas of higher concentration to areas of lower concentration, driven by the tendency to achieve a state of equilibrium.
Temperature
Temperature has a significant impact on the rate of diffusion. As temperature rises, the kinetic energy of molecules also increases—this increase in kinetic energy results in more rapid and energetic movement of molecules.
The increased kinetic energy and collision frequency lead to a higher diffusion rate. Molecules are more likely to move from areas of higher concentration to lower concentration, as they have the energy to overcome potential energy barriers.
Random Molecular Motion
The motion of particles in a fluid (liquid or gas) is random and results from thermal energy. This random motion leads to collisions between particles, causing them to move from regions of higher concentration to regions of lower concentration.
Passive Process
Diffusion is a passive process that does not require energy input from the cell or its surroundings. It is driven solely by the kinetic energy of particles and the natural tendency of systems to reach a state of equilibrium.
Examples of Diffusion in Biology
- Cellular Membranes: Substances such as oxygen, carbon dioxide, and small lipophilic molecules can diffuse across cell membranes.
- Intracellular Processes: Diffusion is involved in the movement of ions and molecules within the cytoplasm of cells.
- Gas Exchange in Lungs: Oxygen and carbon dioxide diffuse across the alveolar membranes in the lungs during respiration.
- Nutrient Absorption in the Digestive System: Nutrients from the digestive tract can diffuse into the bloodstream.
Osmosis
Osmosis is a specific type of diffusion involving the movement of water molecules across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration.
Equilibrium
Equilibrium is reached when the concentration of particles is uniform throughout the system. While individual particles continue to move, there is no net movement in any particular direction.
Related Links
Cytoplasm
Molecules
Solvent
Solution