Elements
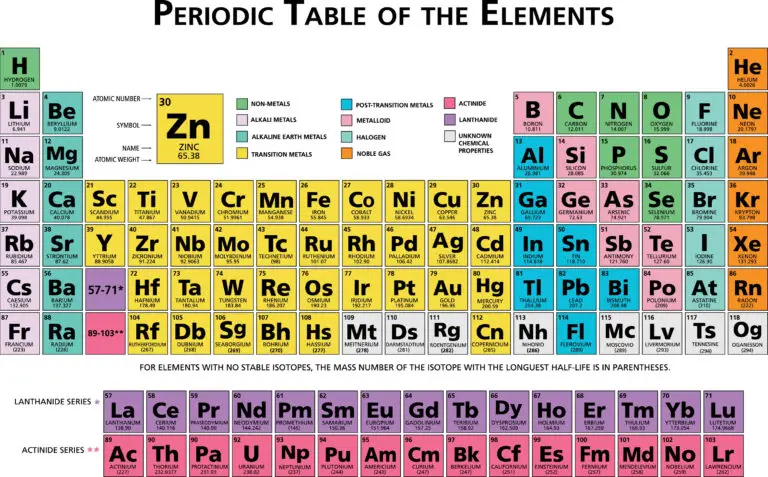
Table of Contents
What are Elements?
In chemistry, an element refers to a fundamental substance of atoms, each containing the same number of protons in their atomic nuclei. These protons give the element its unique identity and properties.
Elements are considered the building blocks of matter because they are the simplest substances and cannot be broken down into simpler substances using normal chemical methods.
Each element is represented by a specific symbol, such as “H” for hydrogen, “O” for oxygen, and “Fe” for iron, and they are organized in the periodic table based on their atomic numbers and properties.
Breaking Down Elements
Atomic Structure
Each element is defined by its atomic number, which is the number of protons found in the nucleus of its atoms. This atomic number is unique to each element and serves as its identifying characteristic.
For instance, hydrogen, represented by the symbol H, has an atomic number of 1. This means that every hydrogen atom contains exactly one proton in its nucleus. The atomic number not only distinguishes one element from another but also determines its placement and properties in the periodic table.
Element Symbols
Elements are given symbols based on their names, usually using the first letter(s) of the name, with the first letter capitalized. For instance, oxygen is denoted by the symbol O, carbon by C, and gold by Au (derived from the Latin word “aurum”).
These symbols are shorthand representations that help scientists and chemists identify and refer to elements more easily in various contexts, such as chemical formulas, equations, and the periodic table.
Isotopes of Elements
All atoms of the same element share the same number of protons in their nucleus, giving them their unique atomic number. However, some atoms of an element may have varying numbers of neutrons, leading to different versions of the element called isotopes.
Isotopes of an element possess identical atomic numbers but differ in their mass numbers, which is the combined total of protons and neutrons in the nucleus. This means that isotopes of an element have the same number of protons but varying numbers of neutrons, resulting in differences in their atomic masses and properties.
Periodic Table
The periodic table is a structured arrangement of elements that is organized according to their atomic number, electron configuration, and chemical characteristics. It consists of rows called periods and columns known as groups or families.
Elements within the same group or family tend to share similar chemical properties and behaviors. This organization helps scientists predict the properties of elements based on their location in the periodic table, making it a valuable tool for understanding and studying the properties and behavior of elements.
Chemical Reactions
Elements can use chemical reactions to create compounds by bonding with other elements. These reactions involve the rearrangement of electrons and the establishment of new chemical bonds.
The behavior of elements in these reactions is influenced by their electronic structure, particularly the arrangement of their electrons and the number of valence electrons they possess.
Valence electrons are the electrons in the outermost energy level of an atom and are crucial for bonding. Elements with similar electronic structures and valence electrons often exhibit similar bonding tendencies and chemical behaviors, leading to predictable patterns in chemical reactions and compound formation.
Classification of Elements
- Metals: Elements typically shiny, malleable, ductile, and good conductors of heat and electricity (e.g., iron, copper, aluminum).
- Nonmetals: Elements that are generally poor conductors of heat and electricity can be gases, liquids, or solids at room temperature (e.g., oxygen, carbon, nitrogen).
- Metalloids: Elements that have properties intermediate between metals and nonmetals and exhibit characteristics of both (e.g., silicon, arsenic, germanium).
- Noble Gases: Elements in Group 18 of the periodic table that are generally unreactive due to their stable electron configurations (e.g., helium, neon, argon).
- Transition Metals: Elements in Groups 3-12 of the periodic table exhibit variable oxidation states and often form colorful compounds (e.g., iron, copper, silver).
- Alkali Metals and Alkaline Earth Metals: Elements in Groups 1 and 2, respectively, that are highly reactive (e.g., sodium, potassium, calcium).
- Halogens: Elements in Group 17 that are highly reactive nonmetals (e.g., fluorine, chlorine, bromine).
Related Links
Electrons
Inorganic
Periodic Table
Protons (Chemistry)