Halogens
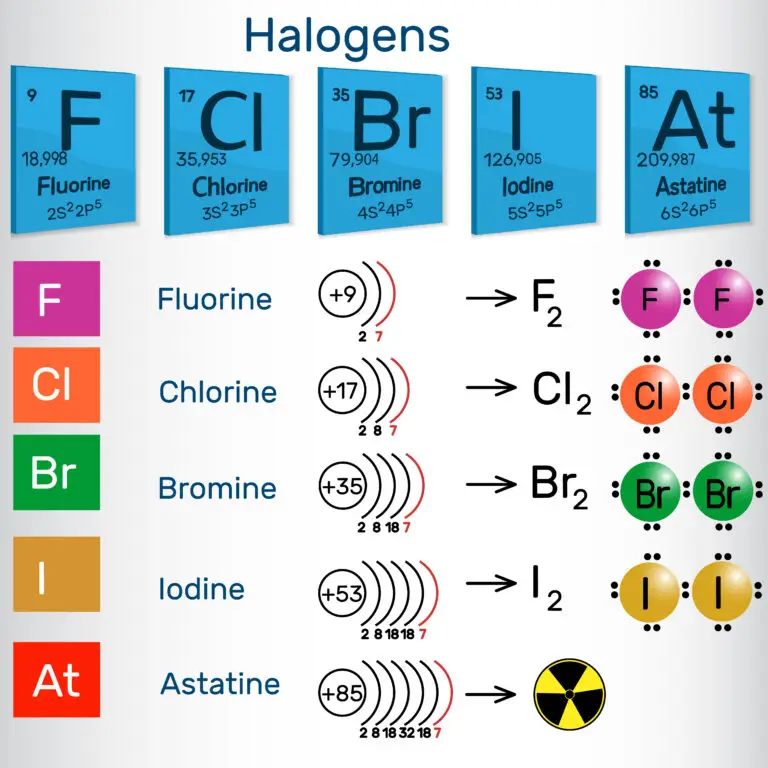
Table of Contents
Halogens (Periodic Table)
Halogens makeup Group 17 of the periodic table, also called the halogen group. These elements are unique because they can create salts by reacting with metals. The name “halogen” comes from the word “salt-former.” This name is apt because halogens often react with metals to form various salts.
The ability of halogens to form salts is crucial in chemistry and our daily lives. For example, when halogens like chlorine or iodine react with metals such as sodium or potassium, they produce common salts like sodium chloride (table salt) or potassium iodide. These reactions are not only fundamental in chemical processes but also play a significant role in many industrial and biological applications.
Characteristics of Halogens
Halogens share several common characteristics:
- They have seven valence electrons in their outermost electron shell, making them highly reactive nonmetals.
- They readily gain an electron to achieve a stable electron configuration, forming -1 ions (anions) known as halides (e.g., fluoride, chloride, bromide, iodide).
- Halogens exhibit increasing atomic size, atomic radius, and melting/boiling points down the group (from fluorine to iodine).
- They can form diatomic molecules (F2, Cl2, Br2, I2) in their elemental form, existing as molecules with two atoms bonded together.
The Halogen Group
Fluorine (F)
Fluorine is the first element in the halogen group and is the most reactive halogen. At room temperature, fluorine appears as a pale yellow gas. Its high reactivity allows it to form compounds with a wide range of elements, including metals, nonmetals, and even noble gases, which are usually unreactive. This reactivity is a defining characteristic of fluorine and sets it apart from other elements.
Fluorine’s reactivity is harnessed in various ways in practical applications. For instance, it is crucial in producing fluoride compounds used in dental care to prevent tooth decay.
Chlorine (Cl)
Chlorine is a greenish-yellow gas we often encounter daily, particularly in water purification and disinfection processes. Its strong bleaching properties make it popular in household and industrial cleaning products. Chlorine’s ability to kill bacteria and other pathogens is why it’s commonly used to keep swimming pools clean and safe. Additionally, it plays a crucial role in treating drinking water, ensuring it is safe for consumption.
On the chemical side, chlorine is versatile, forming ionic compounds called chlorides with metals and covalent compounds with nonmetals. This versatility extends to its use in various industries. For example, chlorine compounds are essential in manufacturing plastics, solvents, and pesticides
Bromine (Br)
Bromine stands out in the periodic table as a reddish-brown liquid at room temperature, making it unique among the nonmetallic elements. Its distinctive color and state are not its only notable features; bromine is also known for its wide range of applications in various fields. It is an essential component in the production of flame retardants, which are crucial for enhancing fire safety in textiles, electronics, and furniture.
Furthermore, bromine’s role extends to the pharmaceutical industry, where it is used in the creation of many medications. In chemical synthesis, bromine is valuable for its ability to participate in different reactions, contributing to the manufacture of various compounds. Besides these uses, bromine compounds, or bromides, have a significant place in photography, where they are used due to their sensitivity to light.
Iodine (I)
Iodine, recognized for its dark purple color, is a solid at room temperature. Unique in its properties, it directly transforms from a solid to a purple vapor when heated, a process known as sublimation. This striking visual change underscores iodine’s distinctive characteristics among elements. Beyond its physical properties, iodine plays a crucial role in human health, particularly in thyroid function, which is vital for producing thyroid hormones.
Iodine’s significance extends to various industries in everyday applications. It is a key ingredient in iodized salt, which helps prevent iodine deficiency in populations. The pharmaceutical sector relies on iodine for manufacturing certain medicines, while the dye industry uses it to create vibrant colors. Additionally, iodine compounds are effective disinfectants, commonly used in medical settings to sterilize equipment and surfaces.
Astatine (At)
Astatine is unique among the halogens due to its radioactivity and extreme rarity. It holds the title as the rarest naturally occurring halogen. Because of its scarcity, astatine is mostly produced in minuscule amounts through nuclear reactions, rather than being found in nature. Its radioactivity and short half-life make it challenging to work with and study, leading to limited practical uses.
The applications of astatine are mostly confined to research, particularly in the field of nuclear medicine. Despite its limited use, astatine’s ability to emit radiation is of interest for targeted cancer treatment in theoretical and experimental studies. However, its short half-life means it decays quickly, which complicates its potential applications.
Related Links
Alkaline Earth Metals
Element Groups
Elements
Periodic Table