Heat
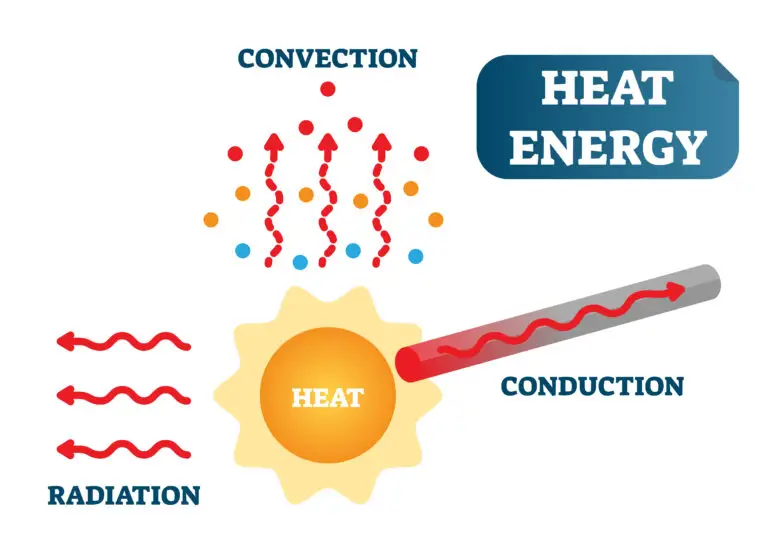
Table of Contents
What is Heat?
Heat is a form of energy linked to the motion and vibration of atoms and molecules in any substance. It arises from the kinetic energy, which is the energy due to motion, of these particles. When particles move faster and collide with each other, they transfer energy in the form of heat. This is why we often feel warmth when there’s a lot of particle motion, for instance, in a heated object.
As the temperature of a substance increases, the kinetic energy of its particles also increases, causing them to move more vigorously. This heightened movement results in more collisions and energy exchanges, leading to an increase in temperature.
How Heat Works
Heat Transfer
Conduction
Conduction is a method of heat transfer that occurs through direct contact between substances or within a single substance. In this process, heat moves from a region of higher temperature to a region of lower temperature without the actual movement of the material. This transfer happens as particles, such as atoms or molecules, vibrate or move and collide with their neighbors, passing on kinetic energy in the process.
The effectiveness of conduction depends on the material’s properties. Some materials, like metals, are excellent conductors of heat because their particles are arranged in a way that allows energy to be transferred quickly between them. For example, when one end of a metal rod is heated, the kinetic energy of the particles at the heated end increases. These particles then collide with adjacent particles, transferring the energy along the rod, making the other end warm.
Convection
Convection is the process of heat transfer that occurs in fluids, which include both liquids and gases. This movement of heat comes from the flow of the fluid itself, where warmer, less dense portions rise, and cooler, denser portions sink. This creates a cycle of circulation that effectively transfers heat throughout the fluid.
The principle behind convection is that when a fluid like water or air is heated, it expands, becomes lighter, and rises because of its lower density compared to the surrounding cooler fluid. As this warm fluid moves away from the heat source, it cools down, becomes denser, and sinks, being replaced by warmer rising fluid. This cycle continues, creating a convective current that distributes heat.
Radiation
Radiation is a method of heat transfer that occurs through electromagnetic waves, like infrared radiation, which can travel through a vacuum. This means that, unlike conduction and convection, radiation does not need a physical substance or medium to transfer heat. Instead, energy is emitted in the form of electromagnetic waves that can travel through empty space.
Objects emit radiation based on their temperature; the hotter an object is, the more infrared radiation it emits. This is why we can feel the heat from the Sun on Earth, even though the Sun is millions of kilometers away in the vacuum of space. The heat travels as solar radiation, reaching Earth and warming its surface.
Units of Heat
The joule (J) is the SI (International System of Units) unit for measuring heat energy, as well as other forms of energy. One joule is defined as the amount of energy expended when one newton of force moves an object one meter. In the context of heat, it’s often related to the amount of energy needed to raise the temperature of water; however, the specific heat capacity of water (the amount of energy required to raise one kilogram of water by one degree Celsius) is actually about 4,184 joules, not one joule.
Besides joules, heat energy can also be measured in calories and British thermal units (BTUs). A calorie (cal) is traditionally defined as the amount of heat required to raise the temperature of one gram of water by one degree Celsius. This unit is frequently used in nutrition to express the energy content of foods. One calorie is equivalent to approximately 4.184 joules.
A British thermal unit (BTU) is another unit of heat energy used, especially in the United States, and is defined as the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit. In terms of joules, one BTU is about 1,055 joules.
Specific Heat Capacity
Specific heat capacity, represented by the symbol �c, is a measure of how much heat energy is needed to increase the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin). This property is intrinsic to each material and can vary widely from one substance to another. The specific heat capacity indicates how a substance responds to heat; substances with high specific heat capacities can absorb a lot of heat without a significant change in temperature, while those with low specific heat capacities heat up or cool down more quickly.
Water, for example, has a high specific heat capacity, meaning it can absorb a lot of heat before it starts to get hot. This characteristic of water makes it an excellent coolant and helps regulate temperature in environments like the Earth’s climate system. It also explains why land near large bodies of water tends to have more moderate temperatures compared to inland areas. In contrast, metals typically have low specific heat capacities, so they heat up and cool down more rapidly.
Applications of Heat
Heat is fundamental in a variety of applications, significantly impacting daily life and industrial processes. In cooking, heat transforms food, making it edible, enhancing flavors, or changing textures. The method of heat transfer can vary; for instance, cooking on a stove typically involves conduction, where heat travels directly from the stove to the pot or pan. Baking in an oven, on the other hand, mainly utilizes convection, circulating hot air around the food to cook it evenly.
For heating systems, such as radiators, boilers, and electric heaters, heat provides comfort in homes and workplaces, especially in colder climates. These systems transfer heat to the surrounding environment, raising the indoor temperature to a comfortable level. The method of heat transfer in these systems can include conduction, convection, and sometimes radiation, ensuring that heat is distributed efficiently throughout a space.
In the industrial sector, heat is a critical element in numerous processes. For example, in metalworking, heat is used to melt metals for casting or forging. In the energy sector, generating steam to drive turbines for electricity production involves the heating of water, often in large boilers. Chemical manufacturing processes also frequently require heat to initiate or accelerate reactions, demonstrating the versatility and necessity of heat in industrial applications.
Related Links
Convection
Energy
Fusion
Homeostasis