Isotopes
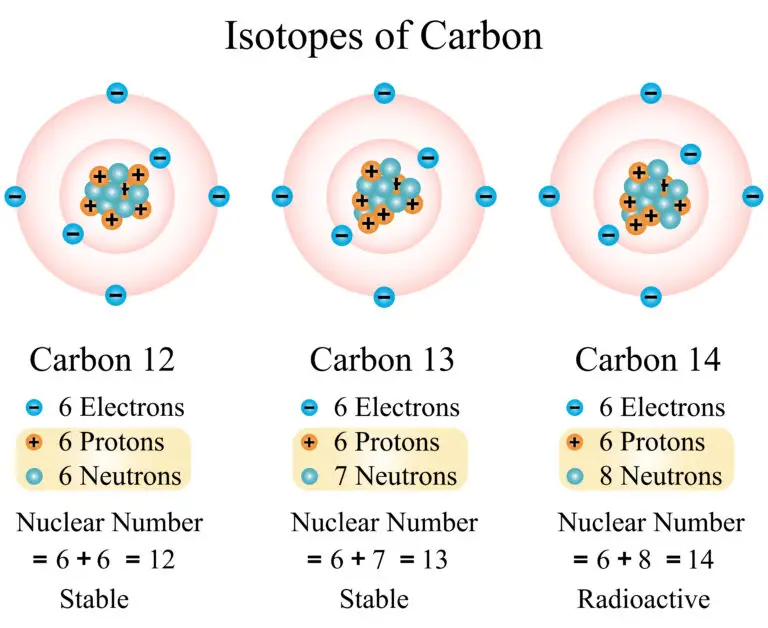
Table of Contents
What is an Isotope?
Isotopes are different forms of the same chemical element, sharing the same number of protons but varying in the number of neutrons within their atomic nuclei. This difference in neutron count means that even though isotopes are the same element, they have different atomic masses. Essentially, the core identity of the element remains unchanged because the number of protons, which determines the element’s type, is constant across its isotopes.
The variation in neutrons affects the mass and physical and chemical properties of the isotopes to some extent. For example, some isotopes may be stable, while others are radioactive and decay over time, releasing energy or particles.
Understanding Isotopes
Composition
Isotopes are forms of the same element with identical numbers of protons but differ in the number of neutrons in their nuclei. This means while the atomic number, which is the number of protons, stays the same for all isotopes of an element, their atomic masses vary due to the different neutron counts. The atomic number is crucial as it defines the element’s identity on the periodic table.
Taking carbon as an example, it has three common isotopes: carbon-12 (12C), carbon-13 (13C), and carbon-14 (14C). All these isotopes contain 6 protons, consistent with carbon’s atomic number. However, they have different numbers of neutrons: 6 in carbon-12, 7 in carbon-13, and 8 in carbon-14, leading to their differing atomic masses.
This variance in neutron number among the isotopes of an element like carbon illustrates the concept of isotopy, showing how isotopes are fundamentally the same element with variations in mass and potentially in their physical or chemical behavior, particularly in the case of radioactive isotopes like carbon-14.
Symbolic Representation
Isotopes are represented using a specific symbolic notation that combines the element’s symbol with its atomic number and atomic mass. The atomic number, which indicates the number of protons, is usually written as a subscript before the element’s symbol, while the atomic mass, which is the total of protons and neutrons, appears as a superscript.
For instance, in the case of carbon isotopes, the notation for carbon-12 is ^{12}C, where 12 (the atomic mass) is the total of protons and neutrons. Similarly, carbon-13 is represented as ^{13}C, and carbon-14 as ^{14}C.
This notation is concise and informative, providing essential details about the isotope at a glance. It helps in identifying the specific isotope of an element and illustrates the differences in atomic mass between isotopes. Despite the variance in mass, the atomic number remains the same, signifying that all these isotopes are forms of the same element, carbon, in this example. This notation system is universally used in scientific literature to clearly and efficiently communicate information about isotopes.
Stable and Radioactive Isotopes
Isotopes can be classified into two main categories based on their stability:
- Stable Isotopes: These isotopes have stable atomic nuclei that do not undergo spontaneous radioactive decay. They are often found in nature and contribute to the normal composition of elements. Examples include carbon-12 and oxygen-16.
- Radioactive Isotopes: These isotopes have unstable atomic nuclei and undergo radioactive decay, emitting radiation in the form of alpha particles, beta particles, or gamma rays to achieve a more stable configuration. Radioactive isotopes are used in various scientific, medical, and industrial applications, such as radiometric dating (e.g., carbon-14 dating), medical imaging (e.g., technetium-99m in nuclear medicine), and nuclear power generation (e.g., uranium-235 and plutonium-239).
Abundance
Isotopes of an element can vary in how frequently they occur in nature, leading to differences in natural abundances. Some isotopes are more common and thus are found in greater amounts than others.
The natural abundance of an isotope refers to how much of it exists compared to all isotopes of that element in a given sample. This abundance is typically expressed as a percentage or a fraction, representing the portion of the element that is made up of that specific isotope.
For example, in the case of carbon, carbon-12 is the most abundant isotope, making up about 98.9% of all carbon found on Earth. Carbon-13 is less abundant, making up about 1.1%, while carbon-14 is extremely rare in comparison.
Effect on Chemical Properties
Isotopes of an element share similar chemical properties because they have the same number of protons and electrons, leading to identical electronic configurations. This similarity in electron arrangement means that isotopes react chemically in much the same way.
However, despite their chemical similarities, isotopes can display differences in physical properties like boiling points, melting points, and densities. These differences arise from the variations in their atomic masses and nuclear stability.
The difference in mass between isotopes affects their behavior in physical processes. For example, heavier isotopes will generally have slightly higher boiling and melting points compared to lighter ones. This is because the greater mass leads to stronger atomic bonds that require more energy to break.
Similarly, the density of an isotope can be influenced by its mass, with heavier isotopes being denser. Additionally, isotopes with unstable nuclei (radioactive isotopes) may exhibit different physical properties due to their tendency to decay and release energy.
Isotopic Analysis
Isotopic analysis is employed across multiple scientific fields, including geology, chemistry, biology, and archaeology, to examine the isotopic composition of different samples. By analyzing the types and amounts of isotopes in a sample, scientists can gain insights into various aspects of the sample’s history and characteristics.
This process involves measuring the ratios of isotopes, which can reveal details about the sample’s origin, age, and chemical makeup, as well as the environmental conditions it was exposed to.
For example, in geology, isotopic analysis helps in understanding the formation and age of rocks and minerals. Archaeology can provide information on the diet and migration patterns of ancient civilizations based on the isotopic signatures in human remains or artifacts.
In environmental science, isotopic analysis can trace pollution sources and study climate change over time by examining the isotopic composition of ice cores or tree rings.
Related Links
Atom
Atomic Number
Mass Number
Neutrons