Mass Number
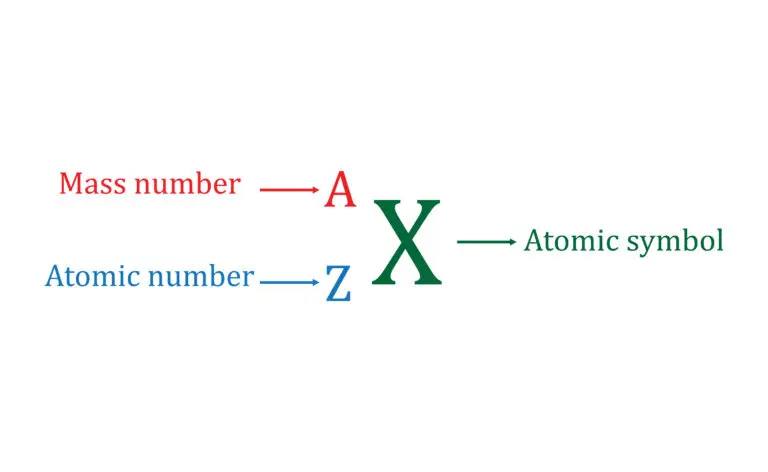
Table of Contents
Mass Number Definition
The mass number of an atom, represented by the symbol A, is the sum of the protons and neutrons in its nucleus. Since protons and neutrons are the heavier components of an atom, their combined count gives the atom’s total mass.
For example, if an atom has 6 protons and 6 neutrons, its mass number would be 12. This number is crucial for identifying an element’s specific isotope because while the number of protons (the atomic number) defines the type of element, the mass number differentiates between the various isotopes of that element, reflecting the differences in their nuclear composition.
Mass Number Explained
Composition
The mass number of an atom is the total count of protons and neutrons in its nucleus. This number signifies the atom’s overall mass, as protons and neutrons make up nearly all of an atom’s weight. Electrons, although part of the atom, are not included in the mass number because their mass is so small it’s considered negligible compared to protons and neutrons.
Therefore, when calculating the mass number, only the protons and neutrons, which contribute significantly to the atom’s mass, are considered. This calculation helps identify and differentiate between an element’s isotopes, as they can have the same number of protons but a different number of neutrons, leading to variations in mass number.
Symbolic Representation
The mass number is usually displayed as a superscript to the left of an element’s symbol to indicate the total number of protons and neutrons in the atom’s nucleus.
For instance, in the case of carbon, which has 6 protons, different isotopes are denoted based on their mass numbers: carbon-12 is written as ^{12}C, where 12 is the mass number (6 protons plus 6 neutrons), carbon-13 is denoted as ^{13}C (6 protons plus 7 neutrons), and carbon-14 is represented as ^{14}C (6 protons plus 8 neutrons).
This notation provides a quick and clear reference to the specific isotope of an element, illustrating both the element’s identity (determined by the number of protons) and its nuclear composition (the total number of protons and neutrons).
Relationship with Atomic Number
The mass number (A) and the atomic number (Z) uniquely define each element’s isotope. The atomic number, Z, represents the number of protons in the nucleus, denoted by a specific symbol for each element. For instance, the atomic number of carbon is 6, indicating it has six protons. The mass number, A, is the nucleus’s total of protons and neutrons.
To find the number of neutrons (N) in an atom, you subtract the atomic number (Z) from the mass number (A), using the formula N=A−Z. This calculation is fundamental in understanding the structure of an atom’s nucleus. For example, with carbon-14 (^{14}C), the atomic number (Z) is 6, and the mass number (A) is 14. Therefore, the number of neutrons (N) would be 14−6=8. This process helps in identifying and distinguishing between the different isotopes of an element, providing a clear picture of their nuclear composition.
Isotopes and Mass Number
Isotopes of an element differ in their mass numbers because they have varying numbers of neutrons. Taking hydrogen as an example, it has three isotopes, each with a different mass number. The first isotope, hydrogen-1 (^{1}H), has a mass number of 1, which means it has one proton and no neutrons. The second isotope, deuterium (^{2}H), has a mass number of 2, indicating it contains one proton and one neutron. The third isotope, tritium (^{3}H), has a mass number of 3, consisting of one proton and two neutrons.
These variations in neutron number result in different mass numbers for each hydrogen isotope, even though they all contain the same number of protons and are thus all forms of hydrogen.
Effect on Atomic Mass
The atomic mass of an element is essentially the weighted average of the masses of all its naturally occurring isotopes, taking into account their relative abundances in nature. The mass number of each isotope plays a critical role in calculating this average because it reflects the isotope’s total mass contributed by protons and neutrons in the nucleus.
This average is expressed in atomic mass units (amu) or unified atomic mass units (u), with both terms being interchangeable. The concept of atomic mass is crucial in chemistry and physics as it provides a practical way to represent the mass of an element that considers the distribution of its isotopes.
For example, the atomic mass of carbon is approximately 12.01 amu, reflecting the fact that while most carbon is carbon-12, there is a small but significant amount of carbon-13 and trace amounts of carbon-14 in naturally occurring carbon, each contributing to the average based on their mass and abundance.
Isotopic Notation
Isotopic notation is a way to specify particular isotopes of an element by combining the mass number with the element’s symbol. The mass number, the sum of an atom’s protons and neutrons, is placed as superscripts before the element’s symbol. This notation provides a concise representation of an isotope’s nuclear composition.
For instance, uranium-235 (^{235}U) is an isotope of uranium with a mass number of 235, indicating it has 92 protons and 143 neutrons (since 235-92=143). Similarly, uranium-238 (^{238}U) is another isotope of uranium, but with a mass number of 238, meaning it has 92 protons and 146 neutrons (because 238-92=146).
Although both isotopes have the same number of protons, as they are the same element (uranium), they differ in the number of neutrons, leading to different mass numbers and distinct physical and chemical properties. This difference is particularly significant in nuclear processes and applications, such as nuclear power and radiometric dating.
Nuclear Stability
The stability of an atomic nucleus largely depends on the balance between its protons and neutrons. Isotopes with equal or nearly equal numbers of protons and neutrons are generally more stable. This balance ensures strong nuclear forces that effectively counteract the repulsion between positively charged protons. When the numbers of protons and neutrons are balanced, the nucleus is in a state where these forces are optimized for stability.
However, when there is a significant discrepancy between the number of protons and neutrons, the nucleus may become unstable. This instability can lead to radioactive decay, where the nucleus releases energy and particles to reach a more stable state. Isotopes with too many neutrons compared to protons, or vice versa, often exhibit this type of instability.
For example, heavy elements with large numbers of protons may require even more neutrons than protons to remain stable, due to the increased repulsion forces among the many protons. If this neutron-to-proton ratio is not met, the isotope will likely undergo radioactive decay to achieve stability.
Related Links
Atomic Number
Isotopes
Matter
Protons