Osmosis
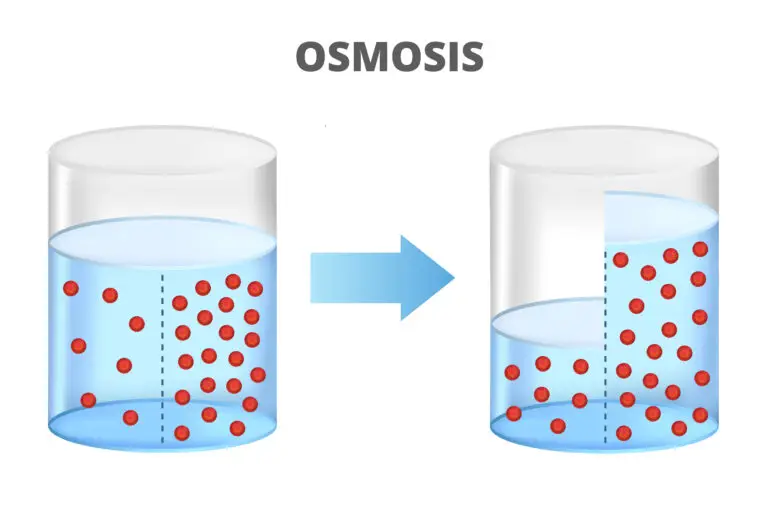
Table of Contents
What is Osmosis?
Osmosis is a biological and physical process involving the movement of solvent molecules, usually water, across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This movement continues until equilibrium is reached, resulting in an equal distribution of solute molecules on both sides of the membrane.
Process of Osmosis
Solute and Solvent
In osmosis, there are two main components: solute and solvent. The solute refers to the dissolved substances (such as salts or sugars), and the solvent is the liquid in which the solute is dissolved. In biological contexts, water is often the solvent.
Selective Permeability
Osmosis occurs across a selectively permeable membrane, which allows the passage of solvent molecules (usually water) while restricting the movement of solute molecules based on their size or charge.
Concentration Gradient
Osmosis involves the movement of solvent molecules from an area of lower solute concentration to an area of higher solute concentration. The concentration gradient across the membrane drives the osmotic flow.
Importance of Osmosis
Cellular Homeostasis: Osmosis helps maintain the balance of water and solutes within cells, ensuring proper cellular function and preventing damage due to swelling or shrinking.
Nutrient Uptake: Osmosis is involved in the uptake of water and nutrients by plant roots from the soil. The movement of water into the roots is driven by osmotic pressure.
Biological Membrane Function: Osmosis is integral to the functioning of biological membranes, influencing processes such as nutrient absorption in the intestines and water reabsorption in the kidneys.
Cell Turgor Pressure: In plant cells, osmosis contributes to turgor pressure, providing structural support and rigidity to the cell.
Preservation Methods: Osmosis is utilized in food preservation methods such as pickling and brining, where the movement of water out of cells helps prevent the growth of spoilage microorganisms.
Related Links
Cellular Respiration
Diffusion
Homeostasis
Turgor Pressure