Periodic Table
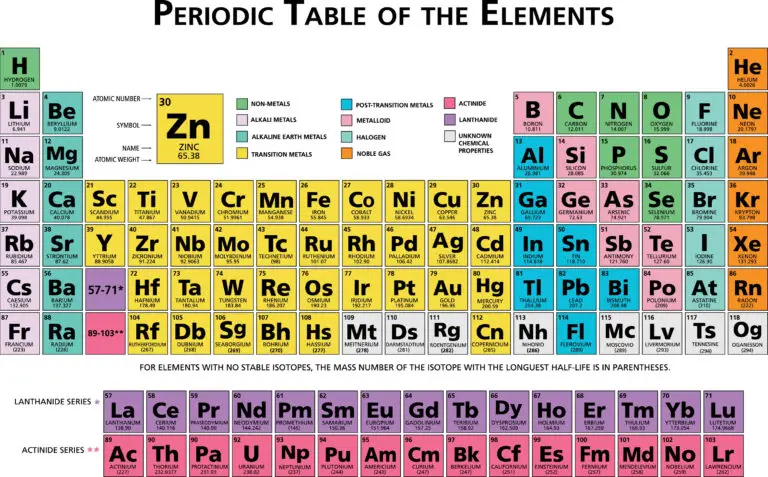
Table of Contents
The Periodic Table
The periodic table arranges chemical elements in a systematic way based on their atomic number, electron configuration, and repeating chemical properties. Elements are placed in rows called periods and columns, known as groups or families. This organization helps to display trends and patterns in both physical and chemical properties across the different elements.
As you move from left to right across a period, elements exhibit a gradual change in properties, such as increased electronegativity, ionization energy, and a decrease in atomic size. Conversely, moving down a group, elements often show similarities in chemical behavior, such as valence electron configuration, resulting in similar reactivity patterns. This structure makes the periodic table a fundamental tool in chemistry, allowing scientists to predict the behavior of elements and their compounds, understand the structure of atoms, and explore relationships between different elements.
Understanding The Periodic Table
Organization
The periodic table is structured into periods, which are horizontal rows, and groups or families, which are vertical columns. Each period signifies a sequence of elements that have the same number of electron shells, with the number increasing as you move down the table. This arrangement means that elements in the same period increase sequentially in atomic number and progressively fill their outer electron shells.
Groups or families group together elements that have similar chemical properties, a similarity that arises from having comparable electron configurations, particularly in their outer shells. For example, elements in the same group typically have the same number of valence electrons, which influences their reactivity and the types of chemical bonds they can form.
Periodic Trends
Periodic trends refer to the patterns in properties that elements display within the same period on the periodic table, as one moves from left to right across a period:
Atomic Size: As you progress across a period, the atomic size or radius typically decreases. This decrease is attributed to the increasing nuclear charge, which is due to the addition of more protons in the nucleus of each successive element. The increased positive charge pulls the electrons closer to the nucleus, reducing the size of the atom.
Ionization Energy: The ionization energy, which is the energy required to remove an electron from an atom, tends to rise across a period. This increase is because the atoms have a stronger nuclear attraction due to the higher number of protons. As a result, more energy is needed to remove an electron from the atom, leading to higher ionization energies.
Electronegativity: Electronegativity, or the ability of an atom to attract and bind with electrons, also increases across a period. This is because the atoms have more protons and a higher nuclear charge, enhancing their ability to attract electrons towards themselves. Consequently, elements towards the right end of a period tend to be more electronegative and better at attracting electrons within a chemical bond.
Groups or Families
The vertical columns in the periodic table are known as groups or families. Elements within a group share similar chemical properties, primarily because they have the same number of valence electrons, which are the electrons in the outermost shell of an atom and are crucial for chemical bonding and reactivity. The groups are numbered from 1 to 18, moving from left to right across the table.
Specific groups have distinctive names that reflect the properties of their elements:
Alkali metals (Group 1): These are highly reactive elements, especially with water, and include lithium, sodium, and potassium. They have one valence electron, which they readily lose to form positive ions or cations.
Alkaline earth metals (Group 2): Elements like magnesium and calcium fall into this group. They are less reactive than alkali metals and have two valence electrons, which they tend to lose in chemical reactions.
Halogens (Group 17): This group includes fluorine, chlorine, and bromine, known for their reactivity and ability to form salts with metals. Halogens have seven valence electrons and typically gain one electron to form negative ions or anions.
Noble gases (Group 18): These are inert, non-reactive gases like helium, neon, and argon. They have a full set of valence electrons, making them chemically stable and rarely participating in chemical reactions.
Representative Elements and Transition Metals
The periodic table is primarily divided into two main sections: the representative (or main group) elements and the transition metals. The representative elements encompass groups 1 and 2 on the left side of the table and groups 13 to 18 on the right side.
These elements are characterized by their predictable valence electron configurations, which lead to regular trends in their chemical and physical properties. For example, the alkali metals in Group 1 are all highly reactive and have a single electron in their outermost shell, while the noble gases in Group 18 are inert due to their complete electron shells.
Transition metals, found in groups 3 to 12, have partially filled d electron subshells and exhibit unique properties, such as the ability to form various oxidation states and colored compounds. These metals, including iron, copper, and gold, often play crucial roles in industrial processes and as catalysts in chemical reactions. Unlike representative elements, transition metals have less predictable trends in properties and often show a greater range of behaviors and chemical complexities.
Block Classification
Elements in the periodic table are classified into blocks based on their electron configurations:
- s-Block: Includes groups 1 and 2, which have their outermost electrons in the s orbital.
- p-Block: Includes groups 13 to 18, which have their outermost electrons in the p orbital.
- d-Block: Transition metals located in groups 3 to 12, with their outermost electrons in the d orbital.
- f-Block: Inner transition metals, including the lanthanides and actinides, located below the main body of the periodic table.
Predictive Power
The organization of the periodic table is a powerful tool that enables scientists to predict the properties of elements based on their positions within it. This predictive capability stems from the periodic trends and patterns that arise from the table’s structure, which reflects the elements’ atomic numbers, electron configurations, and recurring chemical properties. As elements are arranged in order of increasing atomic number, their physical and chemical properties exhibit periodic patterns that repeat across each row or period.
This systematic arrangement has been instrumental in the discovery of new elements. Scientists have used the gaps in the periodic table to predict the existence and properties of elements that had not yet been discovered, guiding experimental efforts to locate and identify them. For example, the discovery of gallium, germanium, and other elements was predicted based on the patterns observed in the table, and their actual properties closely matched the predictions.
Furthermore, the periodic table aids in understanding how elements react chemically and form compounds. The similarities in properties within groups or families allow chemists to anticipate how an element will behave in a reaction, what types of compounds it will form, and how stable these compounds will be.
Related Links
Alkali Metals
Alkaline Earth Metals
Element Groups
Period (Periodic Table)