pH
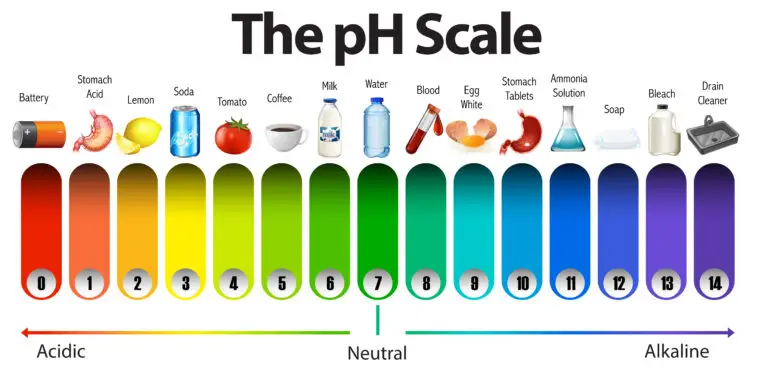
Table of Contents
What is the pH Scale?
pH, short for “potential of hydrogen,” is a scale used to determine how acidic or basic (alkaline) a solution is. It does this by measuring the solution’s concentration of hydrogen ions (H⁺).
The pH scale is set from 0 to 14. Solutions with a pH lower than 7 are acidic, meaning they have a high concentration of hydrogen ions. Solutions with a pH higher than 7 are basic or alkaline, indicating a lower concentration of hydrogen ions and a higher concentration of hydroxide ions (OH⁻). A pH of 7 is neutral, representing a balance between hydrogen and hydroxide ions, with pure water being a classic example of a neutral solution.
This scale is logarithmic, which means each unit change in pH represents a tenfold change in the concentration of hydrogen ions. For instance, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5 and a hundred times more acidic than one with a pH of 6.
How does pH work?
Acidic Solutions
Solutions with pH values below 7 are acidic, characterized by a higher concentration of hydrogen ions (H^+) and a lower concentration of hydroxide ions (OH^-). This imbalance means acidic solutions are more likely to donate protons (H^+) in chemical reactions.
Common examples of acidic substances are lemon juice and vinegar, which have pH values well below 7 due to their high content of citric acid and acetic acid, respectively. Hydrochloric acid (HCl), a strong acid found in stomach acid and industrial solvents, has a very low pH, indicating an even higher concentration of hydrogen ions.
Acids can range from weak, like acetic acid in vinegar, which only partially dissociates in water, to strong, like hydrochloric acid, which dissociates completely, releasing more hydrogen ions into the solution. The acidic nature of these substances makes them reactive and capable of corroding metals, changing litmus paper from blue to red, and neutralizing bases in chemical reactions.
Neutral Solutions
A solution with a pH of 7 is considered neutral, meaning the concentrations of hydrogen ions (H^+) and hydroxide ions (OH^-) in the solution are equal. Pure water at room temperature exemplifies a neutral solution through autoionization, where water molecules dissociate into equal amounts of H^+ and OH^- ions. This reaction can be represented as H_2O ↔ H^+ + OH^-.
In neutral water, the rate at which water molecules dissociate into ions is equal to the rate at which H⁺ and OH⁻ ions recombine to form water molecules, leading to a stable state where the concentrations of both ions are equal. This balance results in a pH of 7, which is the midpoint of the pH scale.
Alkaline (Basic) Solutions
Solutions with pH values above 7 are referred to as alkaline or basic, indicating that they have lower concentrations of hydrogen ions (H^+) and higher concentrations of hydroxide ions (OH^-). In these solutions, the scarcity of H^+ compared to OH^- leads to basic characteristics, such as the ability to accept protons or donate electrons.
Examples of alkaline substances include baking soda (sodium bicarbonate, NaHCO_3), which is commonly used in cooking and as a cleaning agent, and milk of magnesia (magnesium hydroxide, Mg(OH)_2), often used as an antacid and laxative. Soapy water is another example of an alkaline substance; it typically has a high pH because soaps are made of salts of fatty acids, which are basic.
Alkaline solutions can neutralize acids in a chemical reaction to form water and salt. They often feel slippery and can change litmus paper from red to blue.
pH Scale
The pH scale is logarithmic, indicating that each unit change in pH value corresponds to a tenfold difference in hydrogen ion (H^+) concentration. This means that for each whole number decrease in pH, the acidity (or hydrogen ion concentration) increases by a factor of ten. Conversely, for each whole-number increase in pH, the acidity decreases by a factor of ten, making the solution ten times more alkaline or less acidic.
For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4 because it has ten times more hydrogen ions. Similarly, a solution with a pH of 2 would be a hundred times more acidic than a solution with a pH of 4.
pH Measurement
pH can be measured through various methods, including pH meters, pH paper (such as litmus paper), and pH indicators that shift in color at certain pH levels. pH meters are electronic devices that provide accurate and precise measurements of the pH value of a solution by measuring the voltage difference between two electrodes. They are widely used in laboratories and industries where precise pH readings are necessary.
pH paper, on the other hand, offers a quick and simple way to estimate a solution’s pH. When dipped into a solution, the paper changes color based on its acidity or alkalinity, and the resulting color is compared to a reference chart to determine the pH range. Although not as precise as pH meters, pH paper is convenient for fieldwork or quick checks.
pH indicators are chemicals that change color at specific pH values. They can be used in solution form or impregnated in paper strips. Each indicator has a particular pH range over which it changes color, making it useful for determining whether a solution is within that range. Common examples of pH indicators include phenolphthalein, which turns pink in basic solutions, and bromothymol blue, which changes from yellow to blue across a pH range typical of neutral to basic solutions.
Importance in Chemistry and Biology
In chemistry, the pH of a solution can determine the rate at which chemical reactions occur. For example, certain reactions proceed faster in acidic conditions, while others are more efficient in alkaline environments. The solubility of many substances also depends on pH; some compounds are more soluble in acidic solutions, whereas others dissolve better in basic conditions.
In the realm of biology, maintaining a stable pH, known as homeostasis, is vital for the proper functioning of living organisms. For instance, human blood has a narrowly regulated pH around 7.4; even slight deviations from this range can disrupt physiological processes and may lead to serious health issues. The stomach maintains a highly acidic environment (pH 1-3) to aid digestion and kill harmful bacteria. In contrast, the pH of soil can significantly affect plant growth, nutrient availability, and microbial activity, thereby influencing ecosystem health and agricultural productivity.
Related Links
Acid
Bases
Catalyst (Chemistry)
Solubility