Protons (Chemistry)
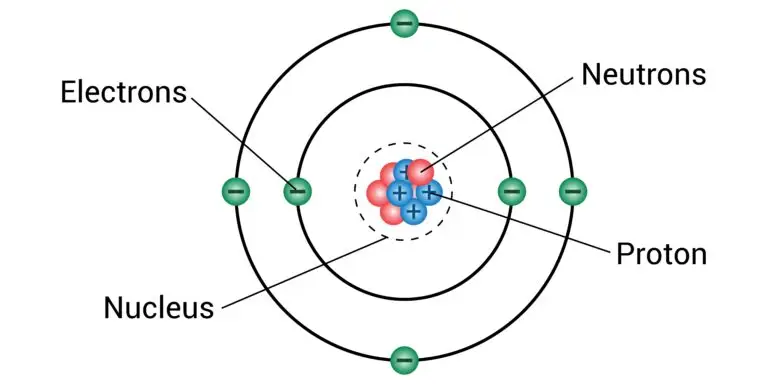
Table of Contents
What are Protons
The number of protons in the nucleus, known as the atomic number, determines an element’s identity; each element has a unique atomic number. For example, hydrogen has one proton, so its atomic number is 1, while carbon has six protons, giving it an atomic number of 6.
Beyond identifying the element, protons also contribute to the atom’s mass and influence its chemical properties. The positive charge of protons balances the negative charge of electrons, resulting in an electrically neutral atom when the number of protons equals the number of electrons. The interaction between protons and electrons defines the atom’s chemical behavior, including how it bonds with other atoms to form molecules.
Protons in the Nucleus
Charge and Symbol
Protons carry a fundamental positive charge conventionally represented as +1. In scientific notation, protons are symbolized by the letter “p” or “p+” to differentiate them from neutral neutrons. The quantity of protons in an atom’s nucleus establishes its atomic number (denoted as Z), which is a defining trait of each element on the periodic table.
The atomic number is crucial because it not only identifies the element but also indicates the number of electrons in a neutral atom, thereby determining the element’s chemical behavior. For instance, carbon has six protons, so its atomic number is 6, which means it also has six electrons in its neutral state. This specific arrangement of electrons around the nucleus, governed by the number of protons, dictates how an element will interact and bond with other elements in chemical reactions.
Mass and Size
The mass of a proton is approximately 1.6726 x 10^{-27} kilograms. A proton is much more massive than an electron, being about 1,836 times heavier. Despite this significant mass, protons are still much smaller than the entire nucleus, which also contains neutrons roughly the same mass as protons.
While protons and neutrons make up almost all of an atom’s mass, they occupy only a small part of its volume, as the nucleus is much denser than the electron cloud surrounding it. Much lighter and less massive, electrons move in the comparatively vast space of the electron cloud, constituting most of the atom’s volume. This contrast in mass and volume distribution between the nucleus (comprising protons and neutrons) and the electron cloud is fundamental to the structure of atoms and how they interact to form molecules and different states of matter.
Role in Atomic Structure
Protons, together with neutrons, form the nucleus at the center of an atom. The specific number of protons within an atom’s nucleus defines its identity as a particular element on the periodic table. For instance, hydrogen atoms are characterized by having one proton, which is the defining feature of hydrogen as an element. Carbon atoms, with six protons, are identified as carbon, and uranium, with 92 protons, is recognized as uranium.
This proton count, or atomic number, not only identifies the element but also influences its chemical properties and behavior. Elements are differentiated and categorized based on their atomic number, meaning that the number of protons is fundamental to the element’s characteristics. Changes in the number of protons transform one element into another, which is a principle used in nuclear reactions and transmutations. Thus, protons are central to the structure of atoms and the vast diversity of elements and materials in the universe.
Atomic Number
An element’s atomic number (Z) is directly tied to the number of protons in the nucleus of its atoms. This number is fundamental in classifying and organizing elements within the periodic table.
Each element has a unique atomic number, distinguishing it from others and defining its place in the table. The atomic number identifies the element and indicates its position and group, which are related to the element’s chemical behavior and properties.
Elements with different atomic numbers have different numbers of protons, making them distinct elements with unique chemical and physical properties. For example, oxygen, with an atomic number of 8, has different characteristics and reacts differently in chemical processes than carbon, which has an atomic number of 6.
Charge Balance
In a neutral atom, the number of protons (positively charged) is exactly balanced by the number of electrons (negatively charged), resulting in a net charge of zero. This equilibrium ensures that the atom is electrically neutral, with the positive charges of the protons in the nucleus being offset by the negative charges of the electrons in the surrounding electron cloud.
Ions form when this balance is disrupted, typically through the gain or loss of electrons. When an atom loses one or more electrons, it becomes a cation, carrying a net positive charge because there are more protons than electrons.
Conversely, when an atom gains extra electrons, it becomes an anion with a net negative charge, as the number of electrons exceeds the number of protons. This change in electron count alters the electric charge of the atom, transforming it into an ion and significantly affecting its chemical properties and reactivity.
Interaction with Electrons
Protons are fundamental in shaping the chemical behavior of atoms due to their positive charge, which attracts negatively charged electrons. This attraction is the cornerstone of chemical bonding, enabling atoms to interact and share or exchange electrons, forming molecules.
The number of protons in an atom’s nucleus, which determines its atomic number, directly influences the type and number of chemical bonds the atom can form.The arrangement of protons in the nucleus, along with the distribution of electrons in the outer shells, dictates an atom’s electron configuration, which in turn affects its chemical reactivity and the kinds of bonds it can form.
For example, atoms with a nearly full or nearly empty outer electron shell are often highly reactive, as they tend to gain, lose, or share electrons to achieve a stable electron configuration, leading to ionic or covalent bonding, respectively. Thus, protons are integral to the identity of an element and its chemical interactions and the structure of compounds formed in reactions.
Nuclear Stability
The stability of an atomic nucleus is closely related to the balance between the number of protons and neutrons it contains. A stable nucleus typically has a balanced ratio of protons to neutrons. However, the nucleus may become unstable when the number of protons is too high or too low relative to neutrons. This instability can lead to radioactive decay, a process where the nucleus seeks a more stable configuration by releasing energy and particles.
Unstable nuclei can undergo several types of radioactive decay processes:
Alpha decay: This involves the nucleus emitting an alpha particle, which consists of two protons and two neutrons, reducing the atomic number by two and the mass number by four.
Beta decay: In beta decay, a neutron in the nucleus transforms into a proton, an electron (beta particle), and an antineutrino. This increases the atomic number by one while the mass number remains unchanged.
Positron emission (or beta-plus decay): This process involves a proton converting into a neutron, a positron (the antimatter counterpart of the electron), and a neutrino, decreasing the atomic number by one.
These decay processes help the nucleus reach a state of greater stability by adjusting the number of protons and neutrons to achieve a more balanced nuclear configuration.
Related Links
Elements
Genus
Mass Number
pH