Reactant
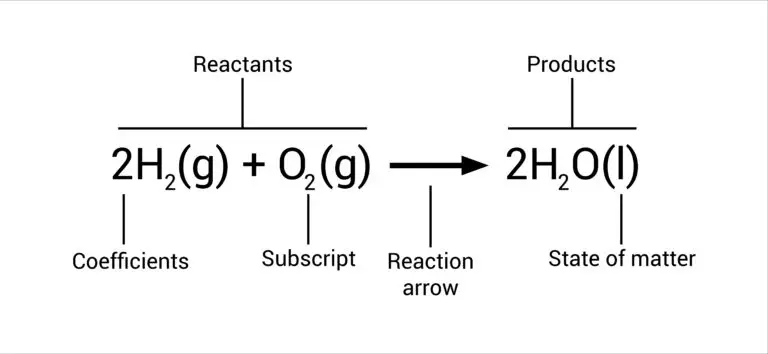
Table of Contents
Reactant Definition
Reactants are the substances present at the start of a chemical reaction and undergo chemical transformations. During a reaction, these substances are consumed as they react, breaking old chemical bonds and forming new ones.
This process leads to the creation of new substances known as products. Reactants are essential in driving the chemical reaction forward, providing the necessary components.
Reactants change their molecular or atomic structures, producing products with different chemical and physical properties compared to the reactants. In a chemical equation, reactants are typically listed on the left side, showing their role as the starting materials for the reaction.
Overview of Reactants
Role in Chemical Reactions
Reactants play a critical role in initiating and driving chemical reactions. They consist of molecules, atoms, or ions that interact with each other, often colliding in a way that allows them to break old bonds and form new ones.
This interaction and rearrangement of atomic structures lead to the creation of new substances known as products. The process involves changes in how atoms are bonded, which can result in significant alterations in physical and chemical properties.
The transformation from reactants to products is the core of chemical processes, where the nature and conditions of the reactants can greatly influence the course and outcome of the reaction. Factors such as the concentration of reactants, temperature, and the presence of catalysts can affect the rate and efficiency of these reactions.
Stoichiometry
In a chemical reaction, reactants are combined in precise proportions determined by the reaction’s stoichiometry. Stoichiometry is the quantitative study of the relationships between the reactants and products in a chemical reaction.
It is based on the balanced chemical equation, which shows the exact ratio in which reactants combine and products are formed. This ratio ensures that atoms are conserved in the reaction, with the same number and type of atoms present in the reactants and products.
Stoichiometry allows chemists to predict how much of each reactant is needed to produce a certain amount of product and determine the reaction yield. The exact amounts of substances involved in reactions can be calculated through stoichiometry, allowing for precise control and optimization of chemical processes.
Conservation of Mass
The law of conservation of mass states that in a closed system, the total mass of the reactants before a chemical reaction equals the total mass of the products after the reaction. This principle underscores that atoms are neither created nor destroyed during a chemical reaction but are merely rearranged to form new substances.
This law is fundamental in chemistry and dictates that all chemical equations must be balanced, with the same number and type of atoms on both sides of the reaction equation. It ensures that the mass remains constant throughout the reaction, providing a basis for quantitative chemical analysis and stoichiometric calculations.
Examples of Reactants
In chemical reactions, the nature of the reactants varies depending on the type of reaction taking place:
Combustion reactions involve reactants that typically include a fuel and an oxidant. For instance, in the combustion of methane (CH₄) with oxygen (O₂), methane and oxygen act as the reactants. The reaction produces carbon dioxide (CO₂) and water (H₂O) as products, releasing energy through heat and light. Combustion reactions are exothermic, meaning they release energy.
Acid-base reactions are characterized by the interaction between an acid and a base. An example is the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH). In this reaction, the acid and base neutralize each other, producing water (H₂O) and a salt, in this case, sodium chloride (NaCl). These reactions are essential in many biological and industrial processes.
Redox (reduction-oxidation) reactions involve the transfer of electrons between reactants. A classic example is the reaction where zinc (Zn) reacts with copper(II) sulfate (CuSO₄). Zinc donates electrons (is oxidized) and displaces copper (which is reduced) from the sulfate compound, resulting in the formation of zinc sulfate (ZnSO₄) and copper metal (Cu). Redox reactions are fundamental to energy production and transfer in biological systems and many industrial applications.
Types of Reactants
Elemental Reactants
Single-element substances comprise only one type of atom and can participate in chemical reactions as reactants. Examples of these substances include hydrogen gas (H₂), oxygen gas (O₂), and iron (Fe). In their reactions, these elements can change to form compounds or interact with other elements.
Compound Reactants
Chemical compounds consist of two or more different elements chemically bonded together, and they can participate as reactants in chemical reactions. Examples include water (H₂O), sodium chloride (NaCl), and glucose (C₆H₁₂O₆).
Water (H₂O) is a compound formed by covalently bonding two hydrogen atoms with one oxygen atom. It is a key reactant in many chemical reactions, including hydrolysis and condensation, and plays a crucial role in biological processes.
Sodium chloride (NaCl), commonly known as table salt, is an ionic compound composed of sodium and chlorine ions. It is formed by transferring an electron from sodium to chlorine, creating a bond due to the attraction between oppositely charged ions. Sodium chloride reacts in aqueous solutions, where it can dissociate into its ions.
Glucose (C₆H₁₂O₆) is a simple sugar and an important energy source in living organisms. It is involved in metabolic processes like cellular respiration and fermentation, where it is broken down to release energy.
Ion Reactants
Ions, charged particles created by the loss or gain of electrons, serve as reactants in various chemical reactions. When atoms lose electrons, they form positively charged ions, or cations, like sodium ions (Na^+). Conversely, when atoms gain electrons, they become negatively charged ions or anions, such as chloride ions (Cl^-). These ions participate in various chemical reactions due to their electric charges, which drive interactions with other ions or molecules.
For example:
Sodium ions (Na^+) are cations that can react with anions to form salts, such as sodium chloride (NaCl), in a reaction with chloride ions.
Chloride ions (Cl^-) are anions that often pair with cations like sodium to form ionic compounds. They play a vital role in reactions such as precipitate formation or neutralization processes with bases.
Hydroxide ions (OH^-), consisting of oxygen and hydrogen, carry a negative charge and are crucial in acid-base chemistry, where they react with acidic protons (H^-) to produce water and salt in neutralization reactions.
Related Links
Catalyst (Chemistry)
Chemical Reaction
Endothermic Reaction
Exothermic Reaction