Solubility
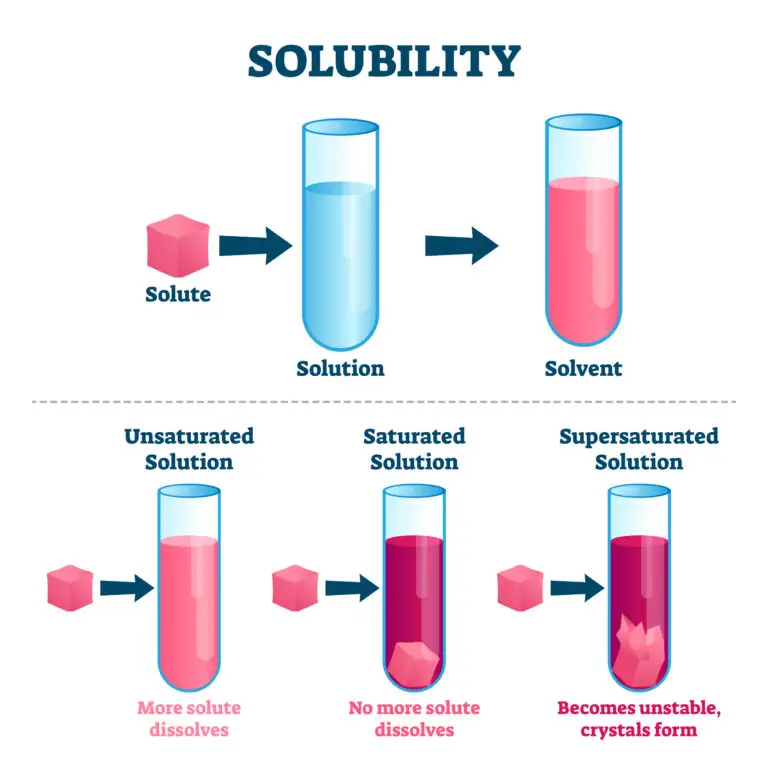
Table of Contents
What is Solubility
Solubility is the maximum quantity of a solute that can dissolve in a specific amount of solvent at a given set of conditions, resulting in a saturated solution. This property is quantitatively expressed in units such as grams per liter (g/L) or moles per liter (mol/L), indicating how much solute can be dissolved in the solvent before reaching a point where no more solute will dissolve under the existing conditions.
Solubility determination is often done through experimental methods and is crucial for understanding how substances interact in solutions. The solubility of a substance can change dramatically with different conditions,
Introduction to Solubility
Factors Affecting Solubility
- Nature of Solute and Solvent: The chemical composition, polarity, and molecular structure of both the solute and solvent influence solubility. Polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes.
- Temperature: In general, the solubility of solid solutes in liquid solvents increases with temperature, as higher temperatures provide more energy for the solute particles to overcome intermolecular forces and mix with the solvent. However, this trend may not apply to all substances, as some solubilities decrease with temperature.
- Pressure: The effect of pressure on solubility depends on the type of solute and solvent. For gases dissolved in liquids (such as carbon dioxide in water), increasing pressure typically increases solubility due to the gas molecules being forced into the liquid phase. However, pressure has minimal effect on the solubility of solid or liquid solutes in a solvent.
Solubility Rules and Guidelines
Like dissolves like: This principle states that substances with similar polarity tend to dissolve in each other. Polar solutes, which have an uneven electron density distribution leading to partial positive and negative charges, dissolve well in polar solvents, which also have uneven electron distributions and partial charges. Conversely, nonpolar solutes, with a more even electron density distribution, are more soluble in nonpolar solvents that lack significant partial charges.
“Oil and water don’t mix”: This common phrase illustrates that nonpolar substances (like oils, fats, and hydrocarbons) do not dissolve in polar solvents like water. This is because the nonpolar molecules cannot form significant interactions with the polar water molecules. However, these nonpolar substances can dissolve in nonpolar solvents, such as hexane or benzene, where the lack of polarity in both the solute and solvent allows them to mix more readily.
Salts and ionic compounds: Many salts and ionic compounds, like sodium chloride (NaCl), are highly soluble in water. This is due to their ionic nature; they dissociate into positive and negative ions in water, forming strong ion-dipole interactions with water molecules. The strong attraction between the water molecules and the ions helps to pull the ionic compound apart and dissolve it.
Units of Solubility
Solubility, the measure of how much of a solute can dissolve in a given solvent, can be quantified in various units tailored to the context of its application:
Grams per liter (g/L): This unit measures solubility as the mass of solute that can be dissolved in a specific volume of solvent (typically water) at a certain temperature. It is a straightforward and practical way to express solubility, especially in laboratory settings where mass and volume are commonly used measurements. For instance, if a substance has a solubility of 20 g/L in water, 20 grams can dissolve in one liter of water under specified conditions.
Moles per liter (mol/L or M): Solubility can also be expressed in molarity, the number of moles of solute per liter of solvent. This unit is particularly useful in chemical calculations involving reaction stoichiometry, as it directly relates to the number of molecules or ions in solution. Molarity provides a clear picture of the concentration of the solution, facilitating the calculation of reactant and product amounts in chemical reactions.
Applications of Solubility in Daily Life
Solubility plays a pivotal role in chemistry and has wide-ranging applications across various sectors:
Pharmaceutical industry: Understanding the solubility of drugs and their active ingredients is fundamental in the pharmaceutical field. It affects how medications are formulated and determine each drug’s most effective delivery method. Solubility influences the drug’s absorption, distribution, metabolism, and excretion in the body, which are crucial factors in achieving the desired therapeutic effect.
Environmental science: In environmental studies, solubility is key to understanding how pollutants and contaminants behave in water and other natural media. The solubility of substances can affect their distribution, persistence, and potential impact on ecosystems and human health. Assessing the solubility of pollutants helps predict their movement, bioavailability, and risk to the environment and living organisms.
Chemical processes: In industrial and chemical engineering, controlling solubility is essential for optimizing various processes, including chemical synthesis, material processing, and waste treatment. In food processing, solubility influences the production and stability of products like beverages, gels, and confectionery. Solubility principles are applied to extract and refine metals in mining and metallurgy. In the manufacturing sector, solubility is critical for designing processes like crystallization, precipitation, and extracting specific compounds.
Related Links
Chemical Compound
Molecule
Solute
Solvent