Solution (Chemistry)
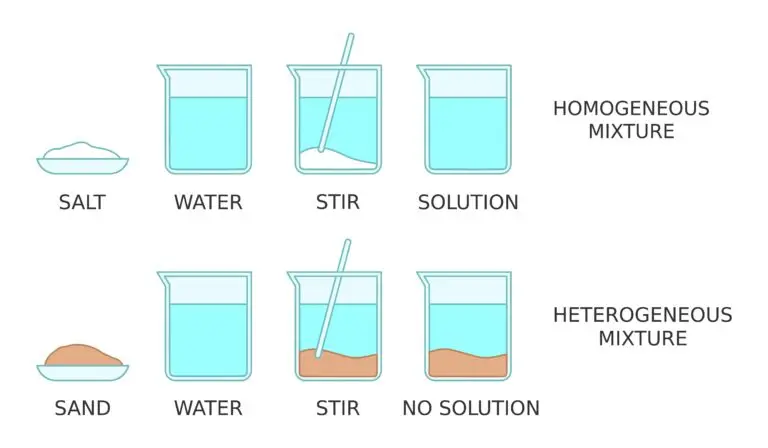
Table of Contents
Chemical Solutions
A solution is a homogeneous mixture in which a solute is evenly dispersed within a solvent. This results in a single-phase system where the solute’s particles are uniformly distributed at the molecular or ionic level.
The solute and solvent can be in any state of matter—solid, liquid, or gas—leading to different solutions based on their physical states. The ability of substances to form solutions and the extent of their solubility depends on factors like temperature, pressure, and the nature of the solute and solvent, including their intermolecular forces and polarity.
Solvent-Solute Interactions
Homogeneous Mixture
A solution is a homogeneous mixture in which the components are uniformly mixed at the molecular or microscopic level. In this mixture, the solute (the substance being dissolved) is completely integrated into the solvent (the substance doing the dissolving). This leads to a consistent and uniform composition and appearance throughout the mixture.
This uniformity means that any sample taken from the solution will have the same proportion of solute and solvent as any other sample of the same volume from the mixture.
Homogeneous mixtures like solutions differ from heterogeneous mixtures, where the components are not uniformly distributed and can often be visually distinguished and physically separated.
Formation of Solutions
The formation of a solution occurs through the process of dissolution or mixing, where solute particles disperse evenly within the solvent. During this process, the solute particles break away from their original structure and become individually surrounded by solvent particles. This interaction is facilitated by forces of attraction between the solute and solvent molecules or ions.
The nature and strength of the solute-solvent interactions are crucial in determining the solubility of the solvent. For instance, in a polar solvent like water, solute particles that are also polar or ionic tend to dissolve well because of the strong ion-dipole or dipole-dipole interactions. Conversely, nonpolar solute particles dissolve better in non-polar solvents due to van der Waals or London dispersion forces more effective in nonpolar environments.
The stability of the resulting solution depends on how well the solute particles are integrated into the solvent and whether the solute-solvent interactions are energetically favorable. Factors such as temperature, pressure, and the presence of other compounds can affect these interactions and, thus, the overall solubility and stability of the solution.
Solubility
Solubility measures how well a solute can dissolve in a solvent under certain conditions, including temperature, pressure, and the specific characteristics of the solute and solvent interactions. This property is crucial in determining how substances mix and form stable solutions. Solubility is not a fixed characteristic; it can change significantly with different conditions and between various substances.
Temperature: The solubility of most solid solutes in liquid solvents increases with rising temperature, as higher temperatures tend to increase the kinetic energy of the molecules, facilitating greater interaction and dissolution. Conversely, the solubility of gases in liquids usually decreases with an increase in temperature.
Pressure: For gases, solubility in liquids is directly influenced by pressure, with higher pressure increasing the gas’s solubility due to the greater number of gas molecules being forced into the solution, as Henry’s law outlines.
Solute-Solvent Interactions: The solute and solvent’s chemical nature and polarity significantly affect solubility. Polar solutes tend to dissolve better in polar solvents due to the strong electrostatic forces between the polar molecules. In contrast, nonpolar solutes are more soluble in nonpolar solvents, where dispersion forces dominate the interactions.
Concentration
The concentration of a solution measures the amount of solute that is dissolved in a given quantity of solvent or total solution volume. It’s a key parameter that describes the strength or intensity of the solution.
Concentration can be expressed in various units, depending on the context and the specific needs of the measurement:
Grams per liter (g/L): This unit specifies the mass of solute in grams dissolved in one liter of solution. It is commonly used in chemistry and biology to describe the concentration of substances in aqueous solutions.
Moles per liter (mol/L or M): Also known as molarity, this unit indicates the number of moles of solute per liter of solution. Molarity is a widely used concentration unit in chemistry because it directly relates to the number of molecules or ions in a solution, which is crucial for stoichiometric calculations in chemical reactions.
Percent by mass (%): This unit expresses the mass of the solute as a percentage of the total mass of the solution. It is often used in industrial formulations, pharmacology, and when preparing mixtures in various fields.
The concentration of a solution significantly influences its physical and chemical properties, such as boiling point, freezing point, viscosity, and reactivity.
Components of a Solution
In a solution, the solute and solvent are the two primary components that interact to form a homogeneous mixture:
Solute
The solute is the component that gets dissolved in the solvent. It can exist in various physical states, such as solid, liquid, or gas, depending on the nature of the solution.
For instance, salt (NaCl) and sugar (sucrose) are common solid solutes that dissolve in water. Gases like oxygen (O₂) and carbon dioxide (CO₂) can also act as solutes when dissolved in liquids, such as water. In addition, various chemicals and compounds, whether ionic or molecular, can serve as solutes in different solvents, contributing to the solution’s properties and applications.
Solvent
The solvent is the medium in which the solute dissolves, usually in greater quantity than the solute. While liquids are the most common solvents (with water being the most universal solvent due to its ability to dissolve many substances), solvents can also be gases or solids.
The major component (nitrogen) acts as the solvent in gas solutions, such as air. Solid solvents exist in solid solutions or alloys, where one metal dissolves another. Common liquid solvents include water, ethanol (an alcohol), acetone, and various organic solvents, each with unique properties that determine which solutes it can dissolve.
Types of Solutions
In a solution, the solute and solvent are the two primary components that interact to form a homogeneous mixture:
Liquid Solutions
Both the solute and the solvent are in liquid form in these solutions. The solubility of one liquid in another can vary widely depending on its chemical properties. Alcoholic beverages, such as wine, where ethanol (the solute) is dissolved in water (the solvent), are common examples of liquid solutions.
Another example is antifreeze, where ethylene glycol, a liquid solute, is dissolved in water to lower its freezing point and raise its boiling point, preventing the coolant in vehicles from freezing or boiling.
Solid Solutions
These are formed when a solid solute is dissolved in a solid solvent, often resulting in an alloy. Alloys are homogeneous mixtures of metals or metals with non-metallic elements.
For instance, brass is an alloy of copper (the solvent) and zinc (the solute), and steel is primarily an alloy of iron (the solvent) and carbon (the solute). The solute atoms, in these cases, are often dispersed uniformly within the crystal lattice of the solvent metal, creating a solid solution that has distinct physical properties compared to the pure metals.
Gas Solutions
In gas solutions, both the solute and the solvent are gases. Air is the most familiar example, being a solution of various gases where nitrogen acts as the solvent, and oxygen, carbon dioxide, and other gases are solutes.
Another example is hydrogen gas dissolved in palladium metal; although the solvent palladium is a solid, this type of solution is often considered under gas solutions due to the gaseous nature of the solute (hydrogen) that diffuses into the solid palladium matrix.
Related Links
Acid
Base (Chemistry)
pH
Redox Reaction