Solvent
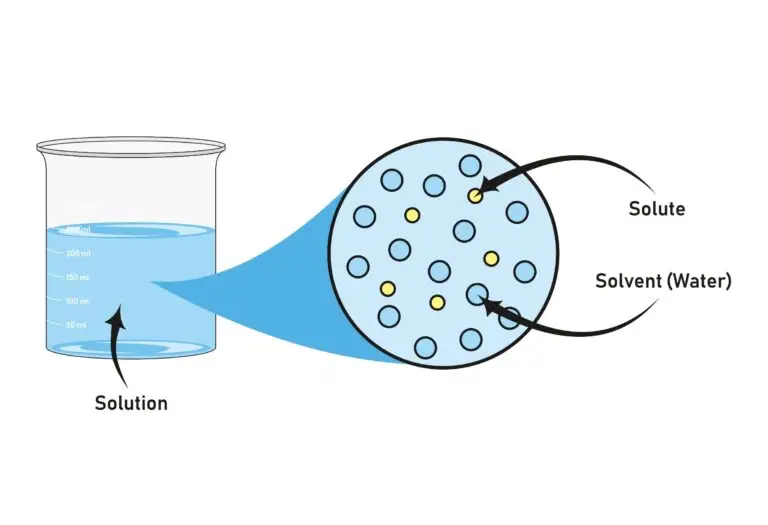
Table of Contents
Solvent Definition
A solvent is a substance in a solution that dissolves the solute, creating a homogeneous mixture known as a solution. While solvents are commonly liquids, they can also be gases or solids, depending on the specific circumstances of the solution. The defining characteristic of a solvent is its capacity to dissolve other substances (solutes), leading to the formation of a solution.
Solvents, including reactions, extractions, and purifications, are critical in chemical processes. They are chosen based on their chemical compatibility with the solute and the desired outcome of the solution process. The solvent’s properties, such as boiling point, polarity, acidity, and reactivity, are essential considerations in its selection to ensure the solute’s effective dissolution and the resulting solution’s stability.
Solvents and Solutions
Role in Solutions
Solvents are fundamental in creating solutions as they provide the environment for solutes to disperse evenly, facilitating the interactions necessary for dissolution. In a solution, the solvent breaks down the solute particles, spreading them uniformly throughout the liquid or other medium. This dispersion is critical for the solute’s molecules or ions to fully integrate into the solution, leading to a homogeneous mixture consistent throughout the composition.
The role of solvents extends beyond just forming solutions; they also play a key part in chemical processes and reactions. Solvents can affect the rate and direction of reactions by influencing the movement of reactants and products, as well as by stabilizing or destabilizing intermediate compounds. Additionally, solvents are used in various industrial and laboratory processes to extract, purify, or transport substances. The choice of solvent is crucial, as it must effectively dissolve the solute without reacting with it or causing unwanted changes.
Properties of Solvents
Solvents exhibit various properties that influence their effectiveness and suitability for various applications. These properties include polarity, boiling point, viscosity, volatility, and chemical compatibility, each playing a crucial role in how a solvent behaves and interacts with solutes:
Polarity: The polarity of a solvent determines the types of solutes it can dissolve. Polar solvents, like water and alcohol, are effective at dissolving polar and ionic substances due to their ability to form electrostatic interactions with the solute. Nonpolar solvents, such as hexane or benzene, dissolve nonpolar substances well, as they can induce dispersion forces that facilitate dissolution.
Boiling Point: The boiling point of a solvent affects its evaporation rate and suitability for processes at different temperatures. Solvents with low boiling points are useful for quick evaporation, while those with high boiling points are preferred for reactions requiring sustained heat.
Viscosity: A solvent’s viscosity can influence the reaction rate and the ease of mixing with solutes. Higher viscosity solvents may slow the reaction rate but can benefit applications requiring controlled delivery or slow release.
Volatility: A solvent’s volatility refers to its tendency to vaporize. Highly volatile solvents are used where quick drying or evaporation is needed, while less volatile solvents are chosen for their stability and prolonged action.
Chemical Compatibility: The solvent must be chemically compatible with the solute, meaning it should dissolve without reacting or causing decomposition.
The selection of a solvent is based on these properties and the specific needs of the application, including the solubility of the solute, the desired concentration of the solution, and the intended use of the solution
Safety Considerations
Safety considerations are paramount when dealing with solvents due to their potential health and safety hazards. These risks can include flammability, toxicity, and environmental impact, necessitating careful handling, storage, disposal, and usage to minimize dangers:
Flammability: Many solvents, especially organic solvents like ethanol and acetone, are highly flammable and pose significant fire risks. Storing these substances away from ignition sources and in well-ventilated areas is crucial to prevent vapor accumulation.
Toxicity: Solvents can also be toxic, either through direct contact, ingestion, or inhalation of their vapors. Toxic effects can range from mild irritation to severe organ damage or even cancer. Using personal protective equipment (PPE), such as gloves and masks, and working in well-ventilated spaces or fume hoods can reduce exposure risks.
Environmental Impact: Some solvents can harm the environment if not disposed of properly. They can contaminate water sources, harm wildlife, and contribute to pollution. It’s important to follow proper waste disposal procedures and consider using “green” solvents, which are more environmentally friendly.
Proper training and adherence to safety protocols are essential when working with solvents to ensure the safety of individuals and the environment. Regulations and guidelines, such as Material Safety Data Sheets (MSDS) for chemicals, provide important information on handling, storage, and disposal practices for various solvents, helping to mitigate risks associated with their use.
Uses and Applications
Solvents have diverse applications across various industries and fields:
- Industrial processes: Solvents are used in manufacturing, cleaning, degreasing, and extraction. For example, organic solvents like acetone and ethanol produce paints, adhesives, and pharmaceuticals.
- Laboratory and research: Solvents are essential in analytical techniques, chromatography, sample preparation, and chemical reactions in laboratories.
- Cleaning and degreasing: Solvents are used as cleaning agents, degreasers, and solvents for removing contaminants, oils, and residues from surfaces and equipment.
- Paints and coatings: Solvents are components in paints, coatings, and inks that help dissolve pigments, provide viscosity control, and facilitate application and drying.
Types of Solvents
Solvents can be classified based on their polarity, ability to donate or accept protons, and the nature of their interactions with solutes. The choice of solvent in a process or reaction depends on the nature of the solute to be dissolved and the desired outcome of the solution’s use, guided by the solvent’s properties and interaction mechanisms.
Polar Solvents
These molecules have significant charge separation, leading to partial positive and negative ends, allowing them to dissolve ionic and other polar substances effectively through dipole-dipole interactions and hydrogen bonding. Water (H₂O) is the most common polar solvent, known as the “universal solvent” for its ability to dissolve many substances. Alcohols like ethanol and polar compounds such as acetone also fall into this category.
Nonpolar Solvents
These solvents have molecules with an even distribution of electrons and no significant charge separation, making them suitable for dissolving nonpolar or hydrophobic substances. They dissolve solutes through van der Waals forces or London dispersion forces. Common nonpolar solvents include hexane, toluene, and carbon tetrachloride (CCl₄).
Aprotic Solvents
Aprotic solvents lack an acidic hydrogen atom, meaning they cannot donate protons in hydrogen bonding. They have a dipole moment and can stabilize positive charges, making them useful in various organic synthesis reactions where strong solvation of cations is required. Examples of aprotic solvents are dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF).
Protic Solvents
Protic solvents contain at least one hydrogen atom connected to an electronegative atom, such as oxygen or nitrogen, which can participate in hydrogen bonding. They can donate protons (H⁺ ions) and effectively dissolve ions and polar molecules. Water, methanol, and acetic acid are protic solvents known for their solvent properties that support various chemical processes and reactions.
Related Links
Chemical Compound
Diffusion
Solubility
Solute