Enzymes: The Catalysts of Life
Enzymes are biological catalysts, or “biocatalysts,” that play a vital role in speeding up the chemical reactions necessary for life. Moreover, found in all living organisms, they enable and regulate processes ranging from digestion to DNA replication. Without these biocatalysts, these reactions would occur far too slowly to sustain life. Additionally, each enzyme is highly specific, usually catalyzing only one type of chemical reaction. By lowering the activation energy required for a reaction to take place, these catalysts allow cells to function efficiently under normal biological conditions. Therefore, understanding how enzymes work and their importance in biological systems is key to fields like medicine, genetics, and biochemistry..
What Are Enzymes?
Biocatalysts, which are proteins, act as catalysts in biological systems. A catalyst speeds up a chemical reaction without being consumed or permanently altered in the process. These catalysts drive nearly every aspect of cellular function, from breaking down food during digestion to synthesizing complex molecules like DNA.
Structure of Biocatalysts:
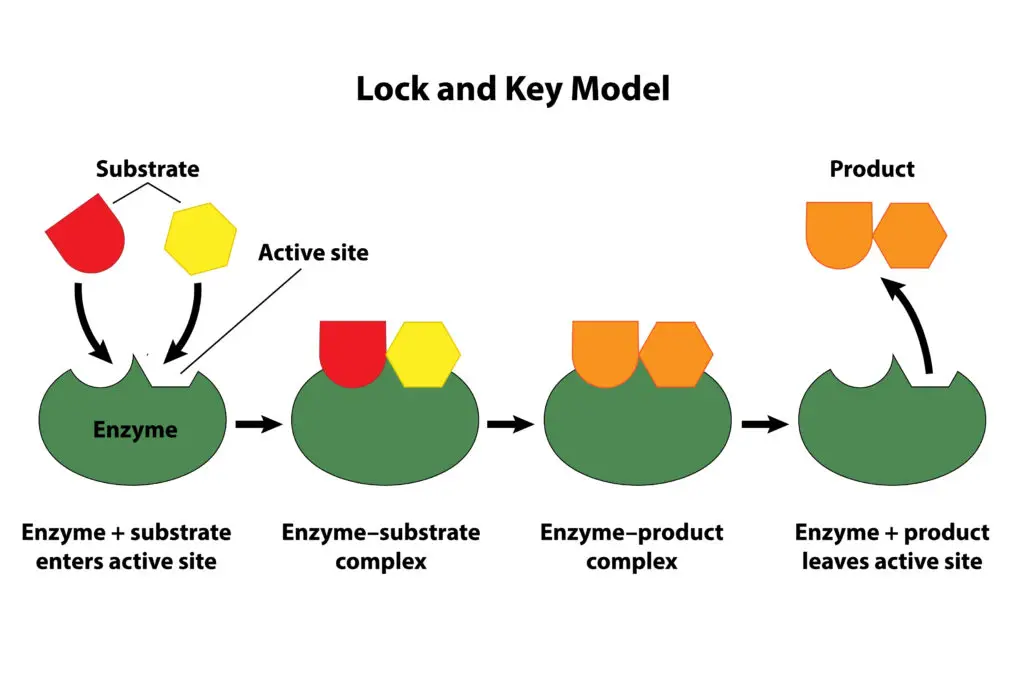
These large, complex proteins consist of chains of amino acids. The specific sequence of amino acids determines the shape and function of each catalyst. As a result, each one has a unique three-dimensional structure, including an active site—the region where the substrate (the molecule the biocatalyst acts on) binds.
The active site is highly specific, allowing only particular substrates to fit, similar to how a key fits into a specific lock. This specificity is a fundamental feature of their function.
How Biocatalysts Work:
These proteins work by lowering the activation energy of a reaction—the energy required for the reaction to proceed. By reducing this barrier, they enable reactions to occur more rapidly and under milder conditions than would be possible without them.
The catalyst binds to its substrate at the active site, forming an enzyme-substrate complex. Once bound, it facilitates the chemical reaction that converts the substrate into products. After the reaction, it releases the products and is ready to catalyze the reaction again.
Importance in Biological Processes
Enzymes are critical to virtually all biological processes. Without them, the complex reactions that sustain life would occur too slowly to be effective.
- Digestion:
- Enzymes are essential for the breakdown of food molecules into nutrients that the body can absorb. For example, the biocatalyst amylase, found in saliva, breaks down starches into simple sugars, while proteases in the stomach and intestines break down proteins into amino acids. Lipases break down fats into fatty acids and glycerol. These digestive enzymes allow the body to efficiently process and absorb nutrients from the food we eat.
- Metabolism:
- Metabolic pathways are a series of chemical reactions that take place within cells to maintain life. Enzymes regulate these pathways by controlling the speed and efficiency of reactions. For example, catalyst involved in glycolysis help convert glucose into energy in the form of ATP (adenosine triphosphate), which cells use to perform various functions.
- DNA Replication and Repair:
- Enzymes are crucial for the accurate replication of DNA, the genetic material of all living organisms. DNA polymerase is a biocatalyst that helps synthesize new strands of DNA by adding nucleotides to the growing DNA chain. Other catalyst, like helicase and ligase, assist in unwinding the DNA helix and joining DNA fragments, ensuring the integrity of the genetic code during replication and repair.
- Cellular Respiration:
- Enzymes play a key role in cellular respiration, the process by which cells produce energy from nutrients. Specifically, in the mitochondria of cells, enzymes help convert the energy stored in food molecules into ATP. This occurs through a series of reactions, including the citric acid cycle and the electron transport chain.
Factors Affecting Enzyme Activity
Enzyme activity can be influenced by several factors, including temperature, pH, and the concentration of substrates or inhibitors. These factors determine how efficiently the biocatalyst function and can either enhance or inhibit its catalytic abilities.
- Temperature:
- Enzymes function best within a specific temperature range. For most human enzymes, the optimal temperature is around 37°C (98.6°F), which is normal body temperature. If the temperature becomes too high, they can denature, meaning they lose their shape and, as a result, their ability to function. If the temperature is too low, their activity slows down as molecular movement decreases.
- pH:
- The pH level can also affect the catalyst activity. Each enzyme has an optimal pH range within which it operates most effectively. For example, the biocatalyst pepsin, which breaks down proteins in the stomach, works best in the highly acidic environment of the stomach (pH 1.5 to 2). Conversely, amylase in the mouth functions best at a neutral pH (around 7).
- Substrate Concentration:
- The concentration of substrates can affect the rate of enzyme activity. As substrate concentration increases, the rate of reaction increases, up to a point. However, once all enzyme molecules bind to substrates (a state called saturation), the reaction rate levels off because no free enzyme molecules remain to catalyze the reaction.
- Inhibitors:
- Enzyme inhibitors are molecules that reduce or stop catalyst activity. Specifically, competitive inhibitors bind to the enzyme’s active site, blocking the substrate from binding. In contrast, non-competitive inhibitors bind to a different part of the catalyst, causing a change in its shape and consequently reducing its activity. Biocatalyst inhibitors can regulate metabolic pathways, but they can also be used as drugs to block harmful enzymes in the treatment of diseases.
Enzymes in Medicine Industry
The study and application of enzymes extend beyond biology, influencing fields such as medicine, biotechnology, and industry.
- Medical Applications:
- Enzymes are used in medical diagnostics and treatments. For example, in blood tests they can help diagnose diseases such as heart attacks by detecting elevated levels of specific enzymes. In the treatment of genetic disorders, enzyme replacement therapy provides patients with enzymes they cannot produce naturally. Biocatalyst like streptokinase are used to dissolve blood clots in stroke patients, reducing the risk of severe complications.
- Industrial Applications:
- Enzymes are widely used in various industries to improve efficiency and reduce environmental impact. In the food industry, lactase is used to break down lactose in dairy products, making them easier to digest for people with lactose intolerance. They also play a key role in the production of biofuels, detergents, and pharmaceuticals.
- Biotechnology:
- Advances in biotechnology have enabled the development of engineered enzymes that are more efficient or tailored to specific industrial processes. Moreover, CRISPR technology, which allows for precise editing of DNA, relies on enzymes like Cas9 to cut DNA at specific locations, thus enabling genetic modification.
Summary
Enzymes are proteins that speed up chemical reactions in living organisms, crucial for processes like digestion and energy production. These biological catalysts have specific shapes, allowing them to interact with particular molecules, known as substrates. By facilitating reactions efficiently, they play a vital role in maintaining cellular function and overall health.