Diffusion
What is Diffusion?
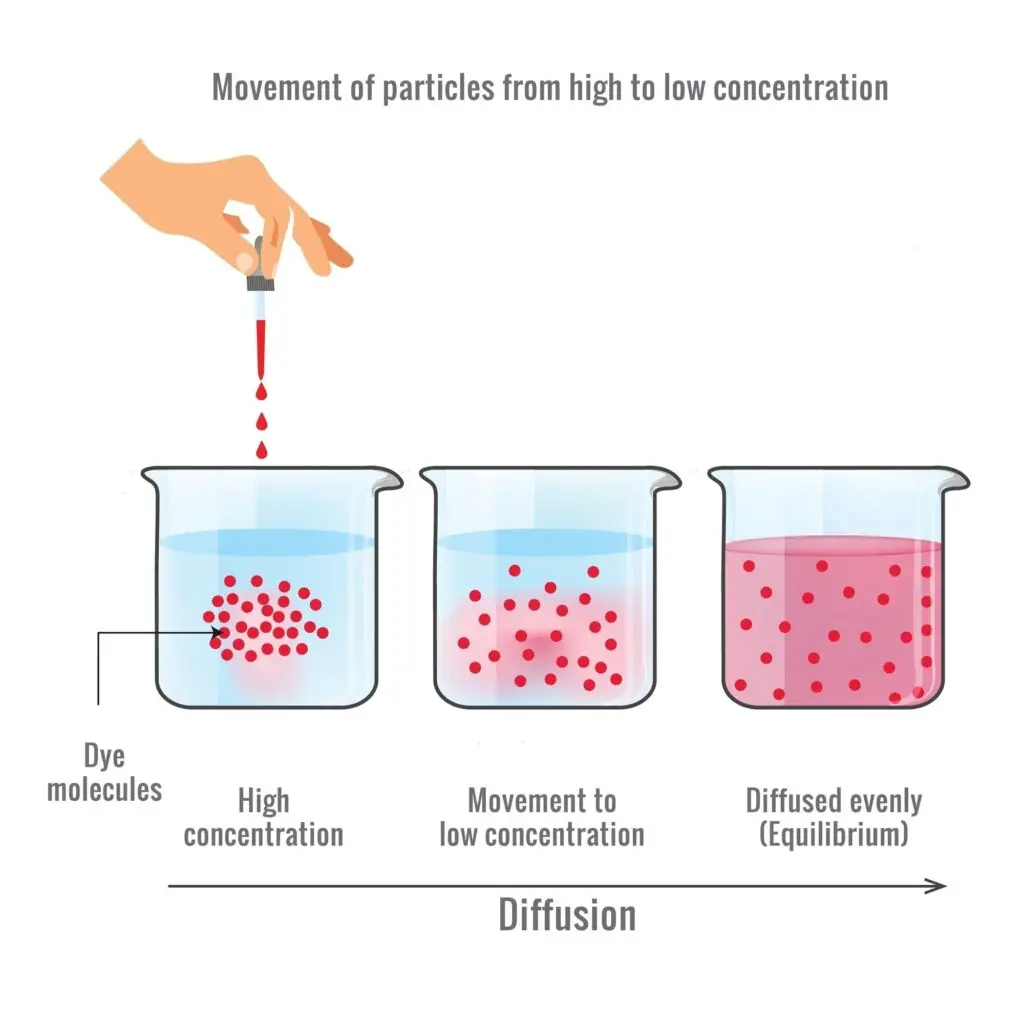
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration, a process that occurs in cells and tissues to help maintain homeostasis within an organism.
Types of Diffusion
Simple diffusion, facilitated diffusion, and osmosis are ways that particles move in and out of cells, each with its own unique process.
In simple diffusion, particles move directly through the cell membrane from an area of high concentration (where there are many particles) to low concentration (where there are fewer particles). They spread out until they are evenly distributed. Simple diffusion doesn’t require any extra help or energy, so it’s very efficient for small particles like oxygen and carbon dioxide.
In facilitated diffusion, particles still move from high to low concentration, but they need a little help to get through the cell membrane. Special proteins in the membrane act as “doors” that allow certain particles to pass through. This type of diffusion is useful for particles that are too large or too charged to pass through on their own, like glucose.
Osmosis is a specific type of diffusion that involves water. In osmosis, water moves from an area with a lot of water (a dilute solution) to an area with less water (a concentrated solution). The goal is to balance the water levels inside and outside the cell. Osmosis is important because it helps cells maintain the right amount of water to stay healthy.
So, simple diffusion is direct, facilitated diffusion uses helper proteins, and osmosis is the movement of water. All three are important for keeping cells balanced and functioning properly.
Real-World Examples
Diffusion happens all around us in everyday life, and you might not even realize it. Here are a few simple examples:
First, think about how a drop of food coloring spreads in a glass of water. At first, the color stays in one spot, but soon it spreads out evenly through the water. This is diffusion, as the color molecules move from an area of high concentration (the drop) to low concentration (the rest of the water).
Another example is the smell of perfume or air freshener in a room. When you spray it, the scent starts strong in one spot, but then spreads throughout the room. Diffusion causes the scent particles to move from the concentrated area near the spray to areas with less scent, so you can eventually smell it everywhere.
Lastly, think about breathing. When you breathe in, oxygen in the air moves into your lungs, where it diffuses into your blood. At the same time, carbon dioxide from your blood diffuses into your lungs and you breathe it out. This process of gas exchange is vital and happens thanks to diffusion.
In all these examples, particles move from areas of high concentration to low concentration, spreading out naturally. Diffusion helps balance things out, making it important both in nature and in our daily lives.