Osmosis
What is Osmosis?
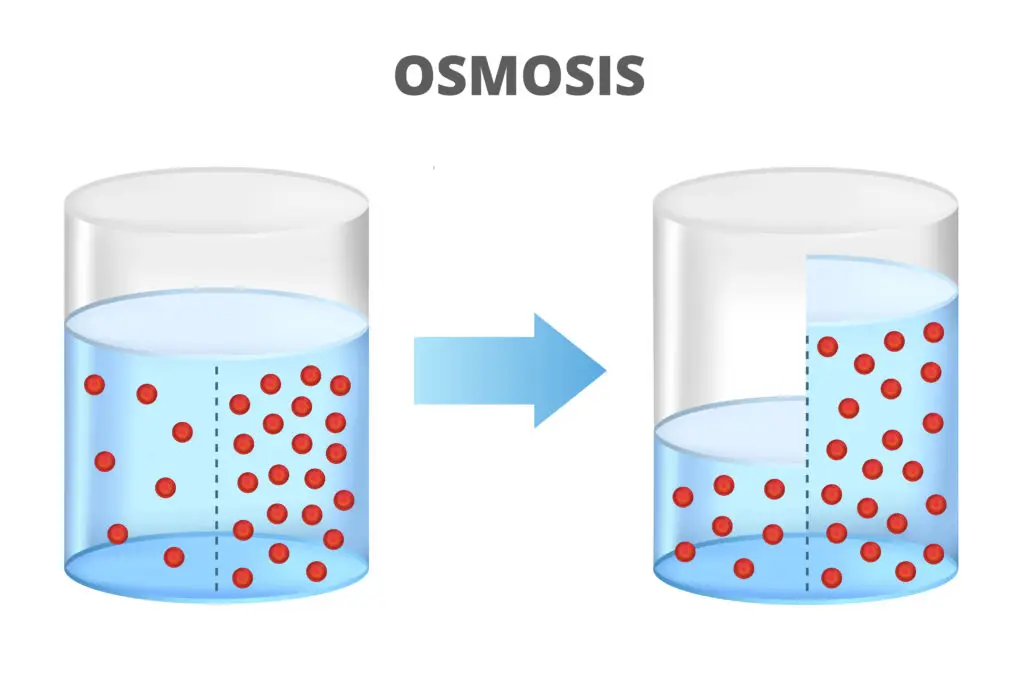
Osmosis is the movement of water molecules across a semi-permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This process helps cells maintain water balance.
Water Movement Across Membranes
Osmosis is the process by which water molecules move across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This movement balances the concentrations on both sides of the membrane. Osmosis is essential for maintaining cellular hydration and regulating the flow of nutrients and waste. Without it, cells couldn’t maintain the proper balance of water needed for survival.
How Osmosis Works
In osmosis, water molecules pass through the cell membrane via protein channels or directly through the lipid bilayer. The direction of water flow depends on the concentration of solutes, such as salts or sugars, inside and outside the cell. For example, if the outside environment has a higher concentration of solutes, water moves out of the cell. Conversely, water flows into the cell when the inside has a higher solute concentration. This process helps cells adapt to changing environments.
Why Osmosis is Important
Osmosis is crucial for maintaining the proper water balance in cells, a condition called osmotic equilibrium. It helps cells absorb water when they’re dehydrated and release excess water when they’re too full. In plants, osmosis supports turgor pressure, which keeps stems and leaves firm. In animals, osmosis maintains fluid balance between cells and their surrounding tissues, ensuring proper functioning of organs and systems.
Role in Everyday Life
Osmosis is at work in many biological processes. For example, when you drink water, osmosis helps it move from your intestines into your bloodstream. In plants, osmosis allows roots to absorb water from the soil, supporting growth and photosynthesis. Even in cooking, osmosis plays a role, like when salt draws water out of vegetables. This everyday importance shows how osmosis connects biology with daily life.
Osmosis and Cell Health
The balance of water through osmosis is critical for cell survival. If too much water flows into a cell, it can swell and burst, a condition called lysis. If too much water leaves the cell, it shrinks, or crenates, affecting its function. Cells use osmosis to regulate their internal environment, relying on mechanisms like ion pumps and aquaporins to control water movement effectively.
Role in Medicine and Research
Osmosis has applications in medical treatments and research. For example, intravenous (IV) fluids are designed with osmotic balance in mind to avoid damaging cells. Dialysis, used for patients with kidney failure, relies on osmosis to remove waste and excess water from the blood. Scientists also study osmosis to understand conditions like dehydration and how cells respond to osmotic stress, improving treatments for related health issues.